6.25: Polymers, Radical Polymerization, Condensation Polymerization, and Polymer Properties
- Page ID
- 408931
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Polymers
A polymer is a long molecule comprised of many (poly) iterations of a molecular unit cell (mer). There are many knobs available to turn to design a polymer with just the right properties. In 3.091, we’ll focus on two types of polymerization reaction: radical and condensation.
Radical polymerization
One mechanism to achieve polymerization is radical polymerization, a chain reaction that is started with the introduction of an initiator with a free radical. The initiator is shown below as \(\mathrm{R}\); more important than its chemistry is the free radical, a highly reactive single electron. The radical polymerization of polyethylene is shown below:
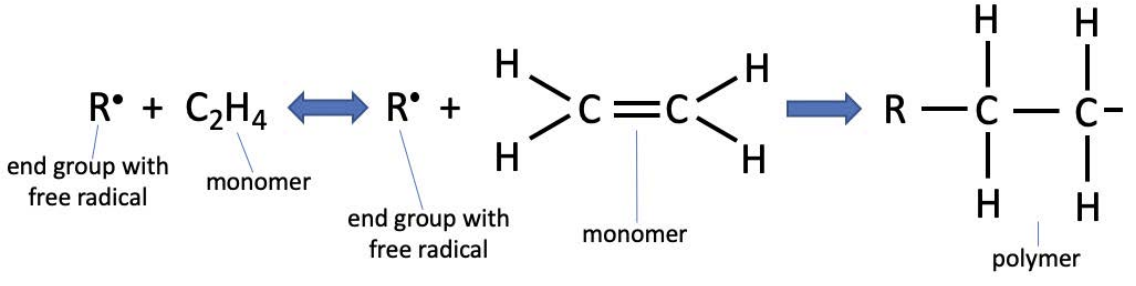
The free radical introduces an extra electron to the monomer, breaking the double bond between the carbon atoms and propagating through to react with another nearby monomer (shown as a half-bond in the polymer above). As long as there is monomer near the end of the chain, the reaction will proceed. Of course, as with any reaction, it is critical that mass is conserved. We can double check that this is the case by counting electrons. The polyethylene monomer has 12 electrons, and with the radical, the system has 13 electrons. In the polymerized picture, there are still 13 electrons.
Radical polymerization works for all sorts of steric groups, where steric refers to the spatial arrangement of atoms in a molecule or side group. For polyethylene, the steric side groups are simply hydrogen atoms; in general, there are limitless options for side groups. Below, the generic reaction shows that what is really important for a radical polymerization to take place is the presence of a carbon=carbon double bond. The
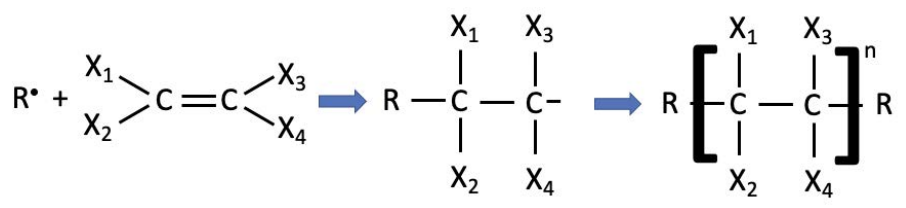
leftmost part of the figure shows a generalized representation of a polymer: there are groups on the end (symbolized as \(\mathrm{R}\), but they don’t necessarily need to be the same), and \(\mathrm{n}\) repeat units that make up the backbone of the polymer.
Condensation polymerization
Condensation polymerization is a type of step-growth reaction that occurs between monomer units with end groups that react to form water as a by-product. The monomers that react could all be the same, or they could be different: this distinction impacts the size of the repeat unit in the polymer that results.

Here, the polymerization reaction mechanism is that the hydroxyl group on the end of one monomer reacts with the hydrogen that terminates the other monomer. Each time this reaction happens, the resulting polymer grows. The polymerization can be halted in many ways; of course, when all of the monomer is consumed or there aren’t monomers near the end of the chain to react, it stops growing. In this case, the hydroxyl group on one end of the monomer comes from the hydroxyl-terminated monomer. The other end could end up being either an \(\mathrm{OH}\) group or a \(\mathrm{H}\) group, depending on what the terminal monomer is- here, it’s drawn assuming an even number of monomers reacted. For each of \(2n\) monomers that add on to the chain, one water molecule is formed as a by-product: this lends the name condensation reaction. Alternatively, a similar process could take place if all of the monomer units are terminated with hydroxyl groups:

The same caveats apply here: there is nothing special about having two different monomers; it could just as easily be a single monomer or many more. The key difference in this case is that there is an extra oxygen on the end of one of the monomers when the water condenses off: this integrates into the backbone as shown, and results in a polymer that is terminated by a hydroxyl group at both ends.
These examples are simply meant as illustrations. With so much flexibility as to the choice of monomer groups, mixtures, and reaction conditions, there is really a whole world of properties that can be engineered.
Polymer properties
The thesis of many efforts in materials science is that structure dictates properties, and polymers are no exception. We have to look at both the chemistry of the monomers and the specific polymerization and processing conditions to understand the electronic and molecular structure, which in turn provide insights as to the micro- and macroscopic properties of the material that results.
Monomer composition: the size and composition of the side groups can play a big role in how tightly a polymer packs. If there are big groups (with a lot of steric bulk), the backbones of the polymer chains can’t sit very close together, resulting in a lower degree of crystallinity.
IMFs: just as the size of the side groups plays a role, the composition can affect how tightly bound neighboring chains are to each other. For example, chains of polyethylene, with just \(\mathrm{H}\) for side groups, slide across each other much easier than chains with side groups that are polar or can form hydrogen bonds.
Backbone structure: depending on how the specific reaction is run, the resulting polymer chains can end up being either linear or branched. Branched polymers pack less densely: you can think of branched polymers as a tangled mess of tree branches, while linear polymers are branches that have been cut into straight pieces and stacked.
Chain length: shorter chains can more easily slide past each other and move around, while long chains get tangled. It’s harder to pull apart a polymer comprised of long chains. If long polymer chains are a tangled mess of cooked spaghetti, short chains are like macaroni. The chain length that results from a given polymerization reaction is called the degree of polymerization, and it is often advantageous to try and engineer the resulting distribution of chain lengths to be as narrow as possible.
Tacticity: the tacticity of a polymer refers to how the side groups are arranged: if all on the same side, it is isotactic. If the groups alternate positions, the polymer is syndiotactic, and if they are randomly arranged, it is atactic.

