0: Introduction
- Page ID
- 474725
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Group theory provides the mathematical framework for applying the symmetry of a chemical structure to characterize its various physical states and properties. Therefore, this section of the course is divided into two subsections:
STRUCTURAL SYMMETRY OF CRYSTALS
- Translational Symmetry: Lattice concept; translation group characteristics and features; periodic boundary conditions; unit cells
- Rotational Symmetry: International notation for point groups; compatibility of rotations and translations; crystallographic point groups; Bravais lattices
- Space Groups: Rotation-translation operations; screw rotations/glide reflections; interpreting space group symbols; symmorphic vs. nonsymmorphic groups; subgroups
ELECTRONIC AND VIBRATIONAL STATES OF CRYSTALS
- Reciprocal Space: Periodic functions and the reciprocal lattice; irreducible representations of the translation group for 1-dimension and generally
- Bloch’s Theorem: Properties of wavefunctions under periodic potentials; Brillouin zones; Bloch functions; electronic and vibrational states for a 1-dimensional crystal
- Irreducible Representations of Space Groups: Relationships between wavevector and rotational symmetry; π electronic structure of graphene

OVERVIEW
Crystalline Structures
Identifying the structural symmetry of molecules and solids is important to understand the nature of their physical and some chemical properties. Molecular symmetry is summarized by a point group, for which all symmetry elements (points, axes, planes) intersect at one fixed point that is assigned as the origin of the spatial coordinate system. For example, consider benzene with point group D6h. The origin lies at the center of the molecule where there is no atom. Some of its symmetry elements include a six-fold rotation axis and six vertical mirror planes; the corresponding operations are rotations by multiples of 2π/6 (60°) and reflections. Crystalline solids, on the other hand, display rotational symmetry at multiple points in space because these structures also exhibit translational periodicity, which is described by a lattice. The combination of rotational and translational symmetry operations yields a space group.
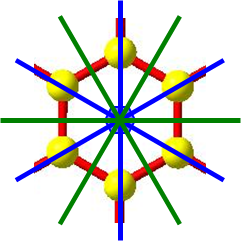
Consider the structure of graphene, which consists of a planar network of fused six-membered rings of carbon atoms. If termination of the structure in the plane is ignored, then there are six-fold rotation axes at the centers of every hexagon, and each carbon atom intersects a three-fold rotation axis. The translational periodicity is represented by unit cells (parallelograms) that connect the centers of every hexagon. As another example, the structure of CeNiC2 contains [NiC2] planes alternating with planes of Ce atoms that sit above and below seven-membered rings of the [NiC2] planes. In the projection of this structure along the stacking direction, a unit cell is a rectangle and vertical mirror planes are apparent. Moreover, there is another type of symmetry operation for this crystalline structure that does not occur for any molecule: glide reflections, in which a reflection through a mirror plane is followed by a displacement parallel to (“gliding along”) the reflection plane. Neither the reflection on its own nor the displacement on its own is a symmetry operation, but the combination of the two operations is for the CeNiC2 structure.

Electronic Structures
The symmetry of atomic structures gives insights into the qualitative features of electronic states, their degeneracies, and allowed or forbidden electronic transitions between states. A thorough understanding of electronic states of crystalline structures requires determining the irreducible representations of the space group. Electronic states may be deduced from spectroscopic measurements or calculated by solving Schrödinger’s equation. The results of these computations for a crystalline solid like graphene are illustrated by energy band diagrams and density of states plots. Energy band diagrams show individual crystal orbital energies plotted as functions of wavevectors, which are quantities related to the translational symmetry. Electronic density of states diagrams illustrate the relative numbers of electronic states vs. energy and are the solid-state analogues of molecular orbital energy diagrams.
STRUCTURAL SYMMETRY OF CRYSTALS
Macroscopic vs. Microscopic Features
(3) Crystalline symmetry reveals itself over length scales ranging from macroscopic to microscopic. Crystals form well-defined faces, sharp edges, and interfacial angles that adopt specific values depending on the overall symmetry of the solid. These features are evident in the figures of pyrite and cinnabar crystals.1 The macroscopic shapes of crystals arise from the microscopic arrangements of the constituent atoms. Amorphous or noncrystalline solids generally lack these macroscopic features, as exemplified by the white material in which the crystals are embedded. Examples of noncrystalline substances include glasses, gels, and some nanostructured materials.
Any atomic structure that repeats periodically in space will give rise to a diffraction pattern obtained using X-ray, electron, or neutron scattering. By analyzing the pattern for the relative locations and intensities of the peaks (spots or lines), the atomic structure of the crystal can be solved. Atomic structure, which is described by bond distances and bond angles based on atomic positions, occurs in real space, whereas diffraction patterns are mapped out in reciprocal space, where distances between points have units 1/length, e.g., 1/Å or 1/nm. Nevertheless, the symmetry of the diffraction pattern in reciprocal space always mimics the symmetry of the atomic (crystal) structure in real space.
Therefore, crystalline symmetry involves two components:
- translational, which is described by a lattice of points that repeat periodically throughout real space; and
- rotational, which is specified by rotation axes and reflection (mirror) planes and is determined by the short-range and long-range organization of atoms of the structure.
These two symmetry components are not mutually exclusive. Taken together, they are used to constitute space groups.
In the mid-1980s, Shechtman et al. reported icosahedral symmetry in electron diffraction patterns for a Mn-Al alloy,2 but this point symmetry cannot occur exactly in a crystal. This discovery elicited much discussion about the types of solid-state structures that give diffraction patterns. Ultimately, this Mn-Al alloy was called a quasicrystal, which is neither crystalline nor amorphous. Since that report, hundreds of quasicrystals have been synthesized and characterized, such as the one shown here, which was prepared in the Ames Laboratory for a Y-Mg-Zn alloy.3 Although most quasicrystals have been prepared in a laboratory, natural quasicrystals have been identified in meteorites, such as icosahedrite (Al63Cu24Fe13)4 and decagonite (Al71Ni24Fe5),5 which show decagonal (10-fold) symmetry.
An important coupling of the macroscopic and microscopic characteristics of symmetry in solids is Neumann’s Principle, which states that the macroscopic (tensor) properties of a crystal have at least the symmetry of the point group of the space group. Another statement of this principle is that if a crystal is invariant with respect to certain rotational symmetry, then any of its physical properties must also be invariant with respect to the same rotational symmetry. The consequences of Neumann’s Principle are significant and emphasize the importance of a thorough structural characterization of matter.
Resources
There are numerous resources covering the subject of group theory applied to chemistry and physics, so please perform your own online searches or browse the University library for the various topics we will cover during this section of the course.
Online Resources
- Bilbao Crystallographic Server (https://www.cryst.ehu.es): A comprehensive resource for widespread applications of space groups; initiated in 1997 at the Materials Laboratory of the University of the Basque Country, Spain.
- International Tables for Crystallography (https://it.iucr.org): A multi-volume set of encyclopedic resources for crystallography; Volume A thoroughly covers two-dimensional plane groups and 3-dimensional space groups – NOTE: use 2006 Edition from the Iowa State University campus.
Monographs
- Space Groups for Solid State Scientists, 3rd Edition, G. Burns and A.M. Glazer, Academic Press, San Diego, 2013 (QC176 .B865 2013); Online access via ISU Library website: Structural symmetry with little mathematical formalism but many black-and-white figures; descriptive style of writing; provides information on how to read and interpret the International Tables for Crystallography, Volume A.
- Physical Chemistry of Solids, H.F. Franzen, World Scientific, Singapore, 1994 (QD478 .F73 1994); Online access via ISU Library website: Group theory for solids is about one-half of the material; mix of formalism and applications; problems at the end of each chapter with solutions as an appendix.
- Applied Group Theory for Chemists, Physicists, and Engineers, A. Nussbaum, Prentice-Hall, Englewood Hills, NJ, 1971 (QD461 .N85): Brings together group theory for molecules and crystalline solids; a nice summary and comparison of notations for point groups; many worked out examples in the midst of descriptions; covers structural aspects and electronic structure.
Additional References
- Induced Representations in Crystals and Molecules, S.L. Altmann, Academic Press, London, 1977 (QD911 .A47): Mathematical treatment of determining irreducible representations for space groups and nonrigid molecules.
- The Mathematical Theory of Symmetry in Solids, C.J. Bradley and A.P. Cracknell, Clarendon Press, Oxford, 1972 (QC176 B65 2010); Online access via ISU Library website: Complete theory of the irreducible representations of the crystallographic point groups and space groups accompanied by extensive tables.
- Elementary Crystallography; An Introduction to the Fundamental Geometrical Features of Crystals, M.J. Buerger, Wiley, New York, 1956 (QD905 .B862e); 3rd Printing 1965 – Full text available via ISU Library website: Broad and detailed geometrical narrative on crystallographic point groups and 3d space groups; derives the 230 3d space groups systematically although the reader must become familiar with Buerger’s notation.
- Group Theory and Electronic Energy Bands in Solids, J.F. Cornwell, North-Holland, Amsterdam, 1969 (QC174.5 C57): Mathematical treatment of determining irreducible representations for space groups and their applications to free-electron energy band structures.
- Group Theory and Its Application to Physical Problems, M. Hamermesh, Addison-Wesley, Reading, MA, 2nd Printing, 1964 (Published by Dover, 1989: QA171 .H28 1989): The “Gold standard” of an extensive mathematical discussion of group theory for various problems involving atoms, molecules, and other chemical systems.
- Crystallographic Groups, T. Janssen, North-Holland, Amsterdam, 1973 (QD905.2 .J36): Succinct but thorough narrative on groups applied to crystals.
- Symmetry Principles in Solid State and Molecular Physics, M. Lax, Dover, Mineola, NY, 2001 (QC174.5 .L38): Another complementary mathematical treatment of group theoretical principles to solid-state problems.
- Crystal Properties Via Group Theory, A.S. Nowick, Cambridge University Press, Cambridge, 1995 (QD911 .N67 1995): Application of group theory to tensorial properties of crystals and crystalline properties.
FOOTNOTES
- 1. Images at http://webmineral.com, David Bethelmy.
- 2. Shechtman, D.; Blech, I.; Gratias, D.; Cahn, J.W. Phys. Rev. Lett. 1984, 53, 1951-1953.
- 3. Fisher, I.R.; Islam, Z.; Panchula, A.F.; Cheon, K.O.; Kramer, M.J.; Canfield, P.C.; Goldman, A.I. Philos. Mag. B 1998, 77, 1601-1615.
- 4. Bindi, L.; Eiler, J.M.; Guan, Y.; Hollister, L.S.; MacPherson, G.; Steinhardt, P.J.; Yao, N. Proc. Natl. Acad. Sci. 2012, 109, 1396-1401.
- 5. Bindi, L.; Yao, N.; Lin, C.; Hollister, L. Amer. Miner. 2015, 100, 2340-2343.


