9.1: Systems Composed of One Reaction
- Page ID
- 357485
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
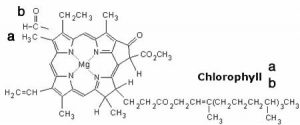
We begin with a few important reactions that can either move backward or forward depending on conditions.[1] Molecular oxygen (\(\mathrm{O}_{2}\)) is a vital component in a number of reactions in our bodies, such as aerobic respiration, the evolutionarily ancient process by which we capture energy from food.[2] \(\mathrm{O}_{2}\) must be transported to every cell so that it can participate in cellular reactions. \(\mathrm{O}_{2}\) diffuses into the bloodstream in the lungs, but it is not very soluble in water (the main component of blood). If we relied on the solubility of oxygen in water to transport it around the body, we would be in trouble. Instead \(\mathrm{O}_{2}\) reacts with (we usually say “binds to”, but this is definitely a chemical reaction) a protein called hemoglobin. The structure of hemoglobin is complex: it is composed of four polypeptide subunits and each polypeptide is associated with a heme group.[3] The heme group contains an iron ion (\(\mathrm{Fe}^{2+}\)) complexed to four nitrogenous bases linked into a ring (called a porphyrin) to form a more or less planar arrangement, as shown in the figure. Heme is also the central active portion of one of the major components of our immune system, myeloperoxidase.[4]When you blow your nose, that familiar green color is actually caused by the light absorbing properties of the heme group in this enzyme, rather than the bacterial infection. Because the heme group is in a different molecular environment, its color appears green rather than red. Chlorophyll, a similar molecule, differs most dramatically from heme in that the iron ion is replaced by a magnesium ion (as shown in the figure). Its function is not to bind \(\mathrm{O}_{2}\) (or \(\mathrm{CO}_{2}\)), but rather to absorb visible light and release an energetic electron as part of the photosynthetic process.

Iron is a transition metal. Recall that these elements have d orbitals, some of which are empty and available for bonding. Iron II (\(\mathrm{Fe}^{2+}\)) has plenty of energetically-available orbitals, and therefore can form Lewis acid–base complexes with compounds that have available electrons (such as nitrogenous bases). Within the porphyrin ring, four nitrogens interact with the \(\mathrm{Fe}^{2+}\) ion. Typically, transition metals form complexes that are geometrically octahedral. In the case of the heme group, four of these interactions involve nitrogens from the four rings; a fifth involves a nitrogen of histidine residue of one of the protein’s polypeptides that approaches from below the ring plane. This leaves one site open for the binding of an \(\mathrm{O}_{2}\) molecule, which has available lone electron pairs.[5] When an \(\mathrm{O}_{2}\) binds to one of these heme groups, \[\text {Hemoglobin } + \mathrm{O}_{2} \rightleftarrows \text { Hemoglobin } - \mathrm{O}_{2} .\]

Note that this way of depicting the reaction is an oversimplification. As we said initially, each hemoglobin molecule contains four polypeptides, each of which is associated with a heme group (green in the figure), so there are four heme groups in a single hemoglobin molecule. Each heme group can bind one \(\mathrm{O}_{2}\) molecule. When an \(\mathrm{O}_{2}\) molecule binds to the heme iron, there are structural and electronic changes that take place within the protein as a whole. This leads to a process known as cooperativity, wherein the four heme groups do not act independently. Binding \(\mathrm{O}_{2}\) to one of the four heme groups in hemoglobin causes structural changes to the protein, which increases the affinity for \(\mathrm{O}_{2}\) in each of the remaining three heme groups. When a second \(\mathrm{O}_{2}\) binds, affinity for \(\mathrm{O}_{2}\) is once again increased in the remaining two heme groups.
As you might suspect, this process is reversible. Imagine a hemoglobin protein with four bound oxygen molecules. When an \(\mathrm{O}_{2}\) is released from the hemoglobin molecule, the affinity between the remaining \(\mathrm{O}_{2}\)'s and the heme groups is reduced, making it more likely that more of the bound \(\mathrm{O}_{2}\)'s will be released. This is an equilibrium reaction, and we can apply Le Chatelier’s principle to it. Where \(\mathrm{O}_{2}\) is in abundance (in the lungs), the reaction shifts to the right (binding and increasing affinity for \(\mathrm{O}_{2}\)). Where \(\mathrm{O}_{2}\) is present at low levels, the reaction shifts to the left (releasing and reducing affinity for \(\mathrm{O}_{2}\)). The resulting hemoglobin molecule has a high capacity for binding \(\mathrm{O}_{2}\) where \(\mathrm{O}_{2}\) is present at high concentrations and readily releases \(\mathrm{O}_{2}\) where \(\mathrm{O}_{2}\) is present at low concentrations. In the blood, [hemoglobin] ranges between \(135–170 \mathrm{~g/L}\), approximately \(2\) millimoles per liter (\(\mathrm{mM}\)), and because there are four \(\mathrm{O}_{2}\) binding sites per hemoglobin, this results in approximately \(\sim 250 \mathrm{~mg/L}\) or \(s8-\mathrm{mM}\) concentration of \(\mathrm{O}_{2}\).
By comparison, \(\mathrm{O}_{2}\)‘s solubility in water is \(\sim 8 \mathrm{~mg/L}\) at \(37 { }^{\circ}\mathrm{C}\), or \(250\) micromoles per liter (\(\mu \mathrm{M}\)). The reaction can be written like this:
\(\mathrm{O}_{2}\) in the air \(\rightleftarrows \(\mathrm{~O}_{2}\) in the \(\text{ blood (liquid) + hemoglobin } \rightleftarrows \text { hemoglobin-}\mathrm{O}_{2} + \mathrm{O}_{2} \text{ in the blood } \rightleftarrows \text { hemoglobin-}2\mathrm{O}_{2} + \mathrm{~O}_{2} \text{ in the blood } \rightleftarrows \text { hemoglobin-}3\mathrm{O}_{2} + \mathrm{O}_{2} \text{ in the blood } \(\rightleftarrows\) \text { hemoglobin-}4\mathrm{O}_{2}
When the hemoglobin reaches areas of the body where \(\left[\mathrm{O}_{2}\right]\) is low, the oxygen dissociates from the hemoglobin into the blood. The dissolved \mathrm{O}_{2} is then removed from the blood by aerobic (oxygen-utilizing) respiration: \[\mathrm{C}_{6}\mathrm{H}_{12}\mathrm{O}_{6} + 6\mathrm{O}_{2} \rightleftarrows 6\mathrm{CO}_{2} + 6\mathrm{H}_{2}\mathrm{O} .\]
The combination of Le Chatelier’s principle and the cooperativity of the \(\mathrm{O}_{2} +\) hemoglobin reaction now leads to the complete dissociation of the \text{ hemoglobin—}4\mathrm{O}_{2}\) complex, releasing \(\mathrm{O}_{2}\). The products of aerobic respiration (essentially a combustion reaction) are carbon dioxide and water. Clearly the water can be carried away in cellular fluid, but the carbon dioxide must be removed in a variety of ways: a small part is removed by reacting with the hemoglobin (but not at the Fe center), some is dissolved in the blood, and some takes part in the buffering system present in the blood, and most is released in the lungs, into the air that you breath out.
Questions
Questions to Answer
- What complicates reaction systems in the real world (outside the lab)?
- Why is \(\mathrm{O}_{2}\) not very soluble in water?
- By what factor does binding with hemoglobin increase solubility of \(\mathrm{O}_{2}\) in water?
- Draw Lewis structures for \(\mathrm{O}_{2}\) and \(\mathrm{CO}\). Why do you think they bind in similar ways to hemoglobin?
- Why does \(\mathrm{CO}_{2}\) react differently with hemoglobin from the way \(\mathrm{O}_{2}\) interacts with hemoglobin?
Questions to Ponder
- Why does it make physiological sense that \(\mathrm{O}_{2}\) binds to oxygen-free hemoglobin (deoxyhemoglobin) relatively weakly and cooperatively ?


