5.3: Vibrating, Bending, and Rotating Molecules
- Page ID
- 355817
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)As we have already seen the average kinetic energy of a gas sample can be directly related to temperature by the equation \(\mathrm{E}k(\mathrm{bar}) = \frac{1}{2} mv(\mathrm{bar})^{2} = \frac{3}{2} k\mathrm{T}\) where \(v(\mathrm{bar})\) is the average velocity and \(k\) is a constant, known as the Boltzmann constant. So, you might reasonably conclude that when the temperature is \(0 \mathrm{~K}\), all movement stops. However, if a molecule stops moving we should be able to tell exactly where it is, right? Oh no! That would violate the uncertainty principle, which means there will need be some uncertainty in its energy! At \(0 \mathrm{~K}\) (a temperature that cannot be reached, even in theory) the system will have what is called zero point energy: the energy that remains when all the other energy is removed from a system (a quantum mechanical concept completely irrelevant to normal life).
https://www.youtube.com/watch?v=FnUGeYkFCCw
For monoatomic gases, temperature is a measure of the average kinetic energy of molecules. But for systems made up of more complex molecules composed of multiple atoms, there are other ways to store energy besides translation (that is, moving through space). In these situations energy added to a system can not only speed up the movement of molecules but also make them vibrate, bend, and rotate (recall we discussed this briefly in Chapter \(4\))(FIG\(\rightarrow\)). These vibrations, bends, and rotations are distinct for each type of molecule; they depend upon molecular shape and composition. Perhaps not surprisingly, they are quantized. This means that only certain packets of energy can be absorbed or released depending on which vibrations or rotations are involved.[8] Because of that, we can use these molecule-specific energy states to identify molecules and determine their structure at the atomic level. Just as we can identify atoms of elements by their electronic spectra (how their electrons absorb and emit photons as they move from one quantum level to another), we can identify molecules by the way they absorb or emit photons as the molecule moves from one vibrational or rotational state to another. Because it takes less energy to move between vibrational states, photons of infrared or microwave frequencies are typically involved in this analysis. This is the basis for infrared spectroscopy, a topic that we will return to in a separate work.
As materials become more complex in structure, more energy is needed to increase their temperature because there are more ways for a complex molecule to vibrate, bend, and rotate; some of the added energy is used up in vibrations and rotations as well as translations. The amount of energy required to raise the temperature of a particular amount of substance is determined by the molecular-level structure of the material. We can do experiments to determine how adding energy to a substance affects its temperature. Although the word heat is sometimes used to describe thermal energy, in the world of physics it is specifically used to describe the transfer of thermal energy from one thing to another. So, we will stick with thermal energy here.
The units of thermal energy are joules (\(\mathrm{J}\)).[9] Thermal energy is the sum of the kinetic and other potential energies of the particles in a system. There are two commonly used measures of how much energy it takes to change the temperature of a substance and, conversely, how much energy a substance can store at a given temperature: specific heat capacity (\(\mathrm{J/g} { }^{\circ}\mathrm{C}\)) and molar heat capacity (\(\mathrm{J/mol} { }^{\circ}\mathrm{C}\)). The specific heat of a substance tells you how much energy is required to raise the temperature of a mass (\(1 \mathrm{~g}\)) of material by \(1 { }^{\circ}\mathrm{C}\); the molar heat capacity tells you how much energy is required to raise the temperature of a mole of particles by \(1 { }^{\circ}\mathrm{C}\). The specific heats and molar heat capacity of a substance depend on both the molecular structure and intermolecular interactions (for solids and liquids, but not gases). Usually, more complex substances have a higher molar heat capacity because larger molecules have more possible ways to vibrate, bend, and rotate. Substances with strong IMFs tend to have higher heat capacities than those with weaker IMFs because energy must be used to overcome the interactions between molecules, rather than make the substance move faster – which increases the temperature.
Heat Capacity and Molecular Structure
It takes \(4.12 \mathrm{~J}\) to raise 1 gram of water \(1 { }^{\circ}\mathrm{C}\) (or \(1 \mathrm{~K}\).) If you add energy to a pan of water by heating it on a stove top energy is transferred to the molecules of water by collisions with the pan, which in turn has heated up from contact with the heating element[10]. The addition of energy to the system results in the faster movement of molecules, which includes moving from place to place, rotating, bending, and vibrating. Each type of movement adds to the overall thermal energy of the material. Although the molecules in a gas very rarely interact with one another, those in a solid and liquid interact constantly. The increase in temperature as a function of added energy is relatively simple to calculate for a gas; it is much more complicated for liquids and solids, where it depends upon molecular structure and intramolecular (within a molecule) as well as intermolecular (between molecules) interactions.
Consider the molar heat capacities and specific heats of water and the hydrocarbon alcohols (which contain an \(\mathrm{-OH}\) group) methanol, ethanol, and propanol. As you can see in the table below, water has an unusually high specific heat, even though it is smaller than the other molecules. Their specific heats are pretty much constant, but their molar heat capacities increase with molar mass.
| Name | Formula | Molar Mass, \(g\) | Molar Heat Capacity
\(\mathrm{J/mol} { }^{\circ}\mathrm{C}\) |
Specific Heat
\(\mathrm{J/g} { }^{\circ}\mathrm{C}\) |
| Water | \(\mathrm{H}_{2}\mathrm{O}\) | \(18\) | \(75.4\) | \(4.18\) |
| Methanol | \(\mathrm{CH}_{3}\mathrm{OH}\) | \(32\) | \(81.0\) | \(2.53\) |
| Ethanol | \(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{OH}\) | \(48\) | \(112\) | \(2.44\) |
| Propanol | \(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{CH}_{2}\mathrm{OH}\) | \(60\) | \(144\) | \(2.39\) |
So an obvious question is, why is the specific heat of water so much higher than that of these alcohols? The reasons for this (apparent) anomaly are:
- Water molecules are smaller so there are more molecules per gram than there are in the larger, more complex substances.
- Each water molecule can form up to four hydrogen bonds, but the alcohols can only form a maximum of two hydrogen bonds each (why is this?). As thermal energy is added to the system some of that energy must be used to overcome the attractive forces between molecules (that is, hydrogen bonds) before it can be used to increase the average speed of the molecules. Because there are more hydrogen bonds forming attractions between water molecules, it takes more energy to overcome those interactions and raise the kinetic energy of the water molecules. The end result is a smaller increase in temperature for the same amount of energy added to water compared to methanol, ethanol, and propanol.
The relatively high specific heat of water has important ramifications for us. About 70% of the Earth’s surface is covered with water. Because of water’s high specific heat, changes in the amounts of solar energy falling on an area between day and night are “evened out” by the large amount of water in the oceans. During the day, the water absorbs much of the energy radiated from the sun, but without a drastic temperature increase. At night, as the temperature falls, the oceans release some of this stored energy, thus keeping the temperature fluctuations relatively small. This effect moderates what would otherwise be dramatic daily changes in surface temperature. In contrast, surface temperatures of waterless areas (like deserts), planets (like Mars), and the Moon fluctuate much more dramatically, because there is no water to absorb and release thermal energy.[11] This moderation of day–night temperature change is likely to be one of the factors that made it possible for life to originate, survive, and evolve on the early Earth. As we go on, we will see other aspects of water’s behavior that are critical to life.
Removing Thermal Energy from a Gas
Now that we have been formally introduced to the concepts of heat, thermal energy, and temperature, we can examine what happens when energy is added or removed from matter. We begin with a gas because it is the simplest form of matter. We can observe a gas system by looking at a sealed container of water vapor. We can reduce the temperature by cooling the walls of the container; as gas molecules collide with the walls, some of their energy is transferred to the wall and then removed by the cooling system. Over time, the average kinetic energy of the molecules (temperature) decreases. We know that all molecules are attracted to one another by London dispersion forces. In the case of water molecules, there are also interactions mediated by the ability to make hydrogen bonds and dipole–dipole interactions. As temperature increases, these relatively weak interactions are not strong enough to keep molecules stuck together; they are broken during molecular collisions. As the temperature drops, and the average kinetic energy decreases, more and more of these interactions persist for longer and longer times. This enables groups of molecules to form increasingly larger and heavier aggregates. Assuming that our container is on the surface of the Earth, molecules fall out or condense out of the gaseous phase to form a liquid. Because the molecules in the liquid are interacting closely with one another, the volume occupied by these aggregates is much smaller than the volume occupied by the same number of molecules in a gas. The density (mass/volume) of the liquid is higher, and eventually these drops of liquid become large enough to feel the effect of gravity, and are attracted towards the Earth. As the drops of liquid fall to the bottom of the container they merge with one another and the liquid phase below separates from the gaseous phase above. The temperature where the liquid phase first appears is the boiling (or condensation) point of the material (for water it is \(100 { }^{\circ}\mathrm{C}\) under atmospheric pressure at sea level). If we continue to remove energy from the system at a fairly slow, steady rate, the temperature will not change until almost all the water vapor has condensed into liquid. Why do you think this is so? It may be easier to think about the reverse process: when water boils, the temperature of the water does not change until almost all the water in the liquid phase has vaporized, even though energy is being added to the system. What is that energy being used for?
Even at temperatures well below the boiling point there are still some molecules in the gaseous phase. Why? Because within the liquid, some molecules are moving fast enough (and are located close enough to the liquid–gas boundary) to break the interactions holding them in the liquid. When they leave the liquid phase, the average kinetic energy of the liquid drops (the molecules that leave have higher than average kinetic energy) and some of the kinetic energy of the escaping molecules is used to break free of the interactions holding them together in the liquid phase. The escaping molecules now have lower kinetic energy. This is the basis of the process known as evaporative cooling. The same process explains how the evaporation of sweat cools your body.
Questions to Answer
- Can you measure thermal energy directly? Why or why not?
- What can we measure changes in? How does that allow us to figure out changes in thermal energy of a system?
- Draw a graph of the change in temperature when equal amounts of thermal energy are added at the same rate to equal masses of water, ethanol, and propanol.
- Does each sample reach the same temperature? Why or why not?
- Plot the temperature change versus time as a sample of water vapor moves from a temperature of \(110 { }^{\circ}\mathrm{C}\) to \(90 { }^{\circ}\mathrm{C}\).
- Draw a molecular-level picture of what the sample looks like at \(110 { }^{\circ}\mathrm{C}\) and \(90 { }^{\circ}\mathrm{C}\). Explain what is happening in each different part of your graph.
- When energy is added to and the water boils, the temperature stays at \(100 { }^{\circ}\mathrm{C}\) until almost all the water is gone. What is the energy being used for?
Questions to Ponder
- What would life be like if we lived on a planet with no water, but instead the oceans were filled with methanol or ammonia (or filled with hydrocarbons as on Titan, a moon of Saturn)?
- After it’s just finished raining, why do pools of water disappear even when the temperature is below the boiling point of water?
- Clouds are made from small droplets of water, why don’t they fall to Earth?
Liquids to Solids and Back Again
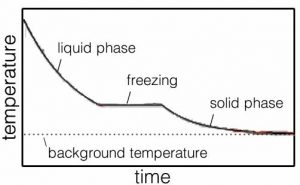
Within a liquid, molecules move with respect to one another. That is why liquids flow. What does that mean at the molecular level? It means that the molecules are (on average) moving fast enough to break some, but not all, of the interactions linking them to their neighbors. But let us consider what happens as we remove more and more energy from the system through interactions of the molecules with the container’s walls. With less energy in the system, there is a decrease in the frequency with which molecules have sufficient energy to break the interactions between them, and as a result interactions become more stable. Once most interactions are stable the substance becomes a solid. The temperature at which the material goes from solid to liquid is termed the melting point. A liquid becomes a solid at the freezing point. For water at atmospheric pressure, this is \(0 { }^{\circ}\mathrm{C}\) (or \(273.15 \mathrm{~K}\)). Just like the boiling/condensation point, the temperature does not change appreciably until all the liquid has solidified into ice, or all the ice has melted (\(\rightarrow\)).
Molecular shape and the geometry of the interactions between molecules determine what happens when water (or any other liquid) is cooled and eventually freezes. In the case of frozen water (ice) there are more than 15 types of arrangements of the molecules, ranging from amorphous to various types of crystalline ice. In amorphous ice, the molecules occupy positions that are more or less random with respect to their neighbors; in contrast the molecules in crystalline ice have very specific orientations to one another. The form of ice we are most familiar with is known as Ice Ih, in which the water molecules are organized in a hexagonal, three-dimensional array. Each molecule is linked to four neighboring molecules through hydrogen bonds. This molecular-level structure is reflected at the macroscopic level, which is why snowflakes are hexagonal. Once frozen, the molecules can no longer move with respect to one another because of the bonds between them; the ice is solid and retains it shape, at both the visible and the invisible (molecular) level. However, because we are not at absolute zero (\(0 \mathrm{K}\) or \(-273.15 { }^{\circ}\mathrm{C}\)), the molecules are still vibrating in place.
Now, what would happen if we heated our container transferring energy from the surroundings into the system (the ice)? As energy is added to the ice the water molecules vibrate more and more vigorously and eventually the hydrogen bonding interactions holding the molecules in place are overcome and the molecules become free to move relative to one another other. The ice melts. At this temperature (\(0 { }^{\circ}\mathrm{C, } 273.15 \mathrm{~K}\)) all the energy entering the system is used to overcome intermolecular attractions, rather than increase the speed of molecular motion. If the system is well mixed, the temperature stays at \(0 { }^{\circ}\mathrm{C}\) until all of the ice has melted. Then the temperature starts to rise again as the water molecules, now free to move relative to each other, increase in kinetic energy.
Because of the arrangement of water molecules in Ice Ih, the hexagonal “cages” of water molecules within the crystal have empty space within them. As the hydrogen bonds break, some of the water molecules can now move closer together to fill in these open spaces. The structure of the ice collapses in on itself. This open network of molecules, which is not present in liquid water, means that Ice Ih is less dense than liquid water, which is why it floats on liquid water. We don’t think much of this commonplace observation, but it is quite rare for a solid to be less dense than the corresponding liquid. More typically, materials (particularly gases, but also liquids and solids) expand when heated as a consequence of the increased kinetic energy, making the particles vibrate more vigorously and take up more space.


