19.6: Commercial Galvanic Cells
- Page ID
- 349773
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To learn how commercial galvanic cells work.
Because galvanic cells can be self-contained and portable, they can be used as batteries and fuel cells. A battery (storage cell)A galvanic cell (or series of galvanic cells) that contains all the reactants needed to produce electricity. is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. In contrast, a fuel cellA galvanic cell that requires a constant external supply of one or more reactants to generate electricity. is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. In this section, we describe the chemistry behind some of the more common types of batteries and fuel cells.
Batteries
There are two basic kinds of batteries: disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; and rechargeable, or secondary, batteries, which form an insoluble product that adheres to the electrodes. These batteries can be recharged by applying an electrical potential in the reverse direction. The recharging process temporarily converts a rechargeable battery from a galvanic cell to an electrolytic cell.
Batteries are cleverly engineered devices that are based on the same fundamental laws as galvanic cells. The major difference between batteries and the galvanic cells we have previously described is that commercial batteries use solids or pastes rather than solutions as reactants to maximize the electrical output per unit mass. The use of highly concentrated or solid reactants has another beneficial effect: the concentrations of the reactants and the products do not change greatly as the battery is discharged; consequently, the output voltage remains remarkably constant during the discharge process. This behavior is in contrast to that of the Zn/Cu cell, whose output decreases logarithmically as the reaction proceeds (Figure 19.4.3). When a battery consists of more than one galvanic cell, the cells are usually connected in series—that is, with the positive (+) terminal of one cell connected to the negative (−) terminal of the next, and so forth. The overall voltage of the battery is therefore the sum of the voltages of the individual cells.
Leclanché Dry Cell
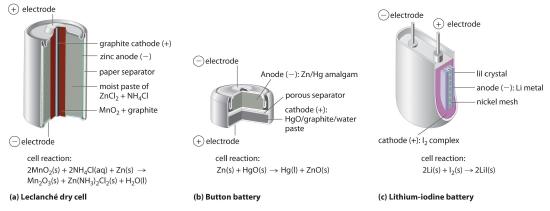
The dry cell, by far the most common type of battery, is used in flashlights, electronic devices such as the Walkman and Game Boy, and many other devices. Although the dry cell was patented in 1866 by the French chemist Georges Leclanché and more than 5 billion such cells are sold every year, the details of its electrode chemistry are still not completely understood. In spite of its name, the Leclanché dry cellA battery consisting of an electrolyte that is an acidic water-based paste containing MnO2, NH4Cl, ZnCl2, graphite, and starch. is actually a “wet cell”: the electrolyte is an acidic water-based paste containing MnO2, NH4Cl, ZnCl2, graphite, and starch (part (a) in Figure 19.5.1). The half-reactions at the anode and the cathode can be summarized as follows:
\( cathode:\; 2MnO_{2}\left (s \right ) + 2NH_{4}^{+}\left ( aq \right ) + 2e^{-} \rightarrow Mn_{2}O_{3}\left ( s \right ) + 2NH_{3}\left (aq \right ) + H_{2}O\left (l \right ) \tag{19.5.1} \)
\( anode:\; Zn\left (s \right ) \rightarrow Zn^{2+}\left ( aq \right ) + 2e^{-} \tag{19.5.2} \)
The Zn2+ ions formed by the oxidation of Zn(s) at the anode react with NH3 formed at the cathode and Cl− ions present in solution, so the overall cell reaction is as follows:
\( overall:\; 2MnO_{2}\left (s \right ) + 2NH_{4}Cl\left ( aq \right ) + Zn\left (s \right ) \rightarrow Mn_{2}O_{3}\left ( s \right ) + Zn\left (NH_{3} \right )_{2}Cl_{2}\left (s \right ) + H_{2}O\left (l \right ) \tag{19.5.13} \)
The dry cell produces about 1.55 V and is inexpensive to manufacture. It is not, however, very efficient in producing electrical energy because only the relatively small fraction of the MnO2 that is near the cathode is actually reduced and only a small fraction of the zinc cathode is actually consumed as the cell discharges. In addition, dry cells have a limited shelf life because the Zn anode reacts spontaneously with NH4Cl in the electrolyte, causing the case to corrode and allowing the contents to leak out.
The alkaline batteryA battery that consists of a Leclanché cell adapted to operate under alkaline (basic) conditions. is essentially a Leclanché cell adapted to operate under alkaline, or basic, conditions. The half-reactions that occur in an alkaline battery are as follows:
\( cathode:\; 2MnO_{2}\left (s \right ) + H_{2}O \left ( l \right ) + 2e^{-} \rightarrow Mn_{2}O_{3}\left ( s \right ) + 2OH^{-}\left (aq \right ) \tag{19.5.4} \)
\( anode:\; Zn\left (s \right ) + 2OH^{-}\left (aq \right ) \rightarrow ZnO \left ( S \right ) + H_{2}O \left ( l \right ) + 2e^{-} \tag{19.5.5} \)
\( overall:\; 2MnO_{2}\left (s \right ) + Zn\left (s \right ) \rightarrow Mn_{2}O_{3}\left ( s \right ) + ZnO \left (s \right ) \tag{19.5.6} \)
This battery also produces about 1.5 V, but it has a longer shelf life and more constant output voltage as the cell is discharged than the Leclanché dry cell. Although the alkaline battery is more expensive to produce than the Leclanché dry cell, the improved performance makes this battery more cost-effective.
Button Batteries
Although some of the small button batteries used to power watches, calculators, and cameras are miniature alkaline cells, most are based on a completely different chemistry. In these batteries, the anode is a zinc–mercury amalgam rather than pure zinc, and the cathode uses either HgO or Ag2O as the oxidant rather than MnO2 (part (b) in Figure 19.13). The cathode and overall reactions and cell output for these two types of button batteries are as follows:
\( cathode \left ( Hg \right ):\; HgO\left (s \right ) + H_{2}O \left ( l \right ) + 2e^{-} \rightarrow Hg\left ( l \right ) + 2OH^{-}\left (aq \right ) \tag{19.5.7} \)
\( overall \left ( Hg \right ):\; HgO\left (s \right ) + Zn\left (s \right ) \rightarrow Hg\left (l \right ) + ZnO \left (s \right ) \;\;\; E_{cell}=1.35 \; V \tag{19.5.8} \)
\( cathode \left ( Ag \right ):\; Ag_{2}O\left (s \right ) + H_{2}O \left ( l \right ) + 2e^{-} \rightarrow 2Ag\left ( s \right ) + 2OH^{-}\left (aq \right ) \tag{19.5.9} \)
\( overall \left ( Ag \right ):\; Ag_{2}O\left (s \right ) + Zn\left (s \right ) \rightarrow 2Ag\left (s \right ) + ZnO \left (s \right ) \;\;\; E_{cell}=1.6 \; V \tag{19.5.10} \)
The major advantages of the mercury and silver cells are their reliability and their high output-to-mass ratio. These factors make them ideal for applications where small size is crucial, as in cameras and hearing aids. The disadvantages are the expense and the environmental problems caused by the disposal of heavy metals, such as Hg and Ag.
Lithium–Iodine Battery
None of the batteries described above is actually “dry.” They all contain small amounts of liquid water, which adds significant mass and causes potential corrosion problems. Consequently, substantial effort has been expended to develop water-free batteries.
One of the few commercially successful water-free batteries is the lithium–iodine batteryA battery that consists of an anode of lithium metal and a cathode containing a solid complex of I2, with a layer of solid LiI in between that allows the diffusion of Li+ ions. The anode is lithium metal, and the cathode is a solid complex of I2. Separating them is a layer of solid LiI, which acts as the electrolyte by allowing the diffusion of Li+ ions. The electrode reactions are as follows:
\( cathode:\; I_{2}\left (s \right ) + 2e^{-} \rightarrow 2I^{-}\left ( LiI \right ) \tag{19.5.11} \)
\( anode:\; 2Li\left (s \right ) \rightarrow 2Li^{+}\left ( LiI \right )+ 2e^{-} \tag{19.5.12} \)
\( overall:\; 2Li\left (s \right ) + I_{2}\left (s \right ) \rightarrow 2LiI\left (s \right ) \;\;\; E_{cell}=3.5 \; V \tag{19.5.13} \)
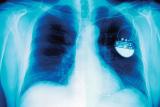
Cardiac pacemaker. An x-ray of a patient showing the location and size of a pacemaker powered by a lithium–iodine battery.
As shown in part (c) in Figure 19.5.1, a typical lithium–iodine battery consists of two cells separated by a nickel metal mesh that collects charge from the anode. Because of the high internal resistance caused by the solid electrolyte, only a low current can be drawn. Nonetheless, such batteries have proven to be long-lived (up to 10 yr) and reliable. They are therefore used in applications where frequent replacement is difficult or undesirable, such as in cardiac pacemakers and other medical implants and in computers for memory protection. These batteries are also used in security transmitters and smoke alarms. Other batteries based on lithium anodes and solid electrolytes are under development, using TiS2, for example, for the cathode.
Dry cells, button batteries, and lithium–iodine batteries are disposable and cannot be recharged once they are discharged. Rechargeable batteries, in contrast, offer significant economic and environmental advantages because they can be recharged and discharged numerous times. As a result, manufacturing and disposal costs drop dramatically for a given number of hours of battery usage. Two common rechargeable batteries are the nickel–cadmium battery and the lead–acid battery, which we describe next.
Nickel–Cadmium (NiCad) Battery
The nickel–cadmiumA type of battery that consists of a water-based cell with a cadmium anode and a highly oxidized nickel cathode., or NiCad, battery is used in small electrical appliances and devices like drills, portable vacuum cleaners, and AM/FM digital tuners. It is a water-based cell with a cadmium anode and a highly oxidized nickel cathode that is usually described as the nickel(III) oxo-hydroxide, NiO(OH). As shown in Figure 19.5.2, the design maximizes the surface area of the electrodes and minimizes the distance between them, which decreases internal resistance and makes a rather high discharge current possible.
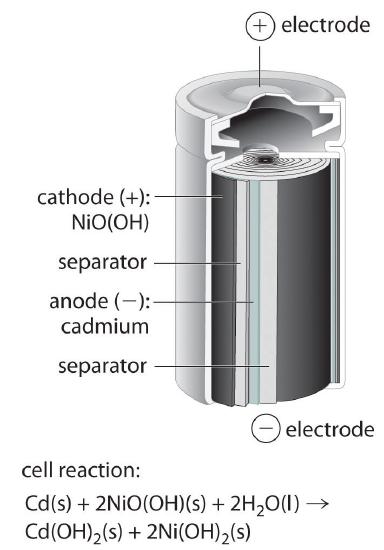
The electrode reactions during the discharge of a NiCad battery are as follows:
\( cathode:\; 2NiO\left ( OH \right )\left (s \right ) + 2H_{2}O\left ( l \right ) + 2e^{-} \rightarrow 2Ni\left ( OH \right )_{2}\left (s \right )+ 2OH^{-}\left ( aq \right ) \tag{19.5.14} \)
\( anode:\; Cd \left (s \right )+ 2OH^{-}\left ( aq \right )\rightarrow Cd\left ( OH \right )_{2} \left ( s \right )+ 2e^{-} \tag{19.5.15} \)
\( overall:\; Cd \left (s \right ) + 2NiO\left ( OH \right )\left (s \right )+2H_{2}O\left ( l \right ) \rightarrow Cd\left ( OH \right )_{2} \left ( s \right ) + 2Ni\left ( OH \right )_{2}\left (s \right )\;\;\; E_{cell}=1.4 \; V \tag{19.5.16} \)
Because the products of the discharge half-reactions are solids that adhere to the electrodes [Cd(OH)2 and 2Ni(OH)2], the overall reaction is readily reversed when the cell is recharged. Although NiCad cells are lightweight, rechargeable, and high capacity, they have certain disadvantages. For example, they tend to lose capacity quickly if not allowed to discharge fully before recharging, they do not store well for long periods when fully charged, and they present significant environmental and disposal problems because of the toxicity of cadmium.
A variation on the NiCad battery is the nickel–metal hydride battery (NiMH) used in hybrid automobiles, wireless communication devices, and mobile computing. The overall chemical equation for this type of battery is as follows:
\( NiO\left ( OH \right )\left ( s \right )+MH \rightarrow Ni\left ( OH \right )_{2}\left ( s \right ) + M\left ( s \right ) \)
The NiMH battery has a 30%–40% improvement in capacity over the NiCad battery; it is more environmentally friendly so storage, transportation, and disposal are not subject to environmental control; and it is not as sensitive to recharging memory. It is, however, subject to a 50% greater self-discharge rate, a limited service life, and higher maintenance, and it is more expensive than the NiCad battery.
Lead–Acid (Lead Storage) Battery
The lead–acid batteryA battery consisting of a plate or grid of spongy lead metal, a cathode containing powdered PbO2, and an electrolyte that is usually an aqueous solution of H2SO4 is used to provide the starting power in virtually every automobile and marine engine on the market. Marine and car batteries typically consist of multiple cells connected in series. The total voltage generated by the battery is the potential per cell (E°cell) times the number of cells. As shown in Figure 19.5.3, the anode of each cell in a lead storage battery is a plate or grid of spongy lead metal, and the cathode is a similar grid containing powdered lead dioxide (PbO2). The electrolyte is usually an approximately 37% solution (by mass) of sulfuric acid in water, with a density of 1.28 g/mL (about 4.5 M H2SO4). Because the redox active species are solids, there is no need to separate the electrodes. The electrode reactions in each cell during discharge are as follows:
\( cathode:\; 2PbO_{2}\left (s \right ) + HSO_{4}^{-}\left ( aq \right ) + 3H^{+}\left ( aq \right ) +2e^{-} \rightarrow PbSO_{4}\left (s \right )+ 2H_{2}O\left ( l \right ) \;\;\; E_{cathode}=1.685 \; V\tag{19.5.17} \)
\( anode:\; PbS \left (s \right )+ HSO_{4}^{-}\left ( aq \right ) \rightarrow PbSO_{4}\left (s \right )+ H^{+}\left ( aq \right ) + 2e^{-} \;\;\; E_{anode}=-0.356 \; V\tag{19.5.18} \)
\( overall:\; 2PbO_{2}\left (s \right ) + 2HSO_{4}^{-}\left ( aq \right ) + 2H^{+}\left ( aq \right ) \rightarrow 2bSO_{4}\left (s \right )+ 2H_{2}O\left ( l \right ) \;\;\; E_{cell}=2.041 \; V \tag{19.5.19} \)
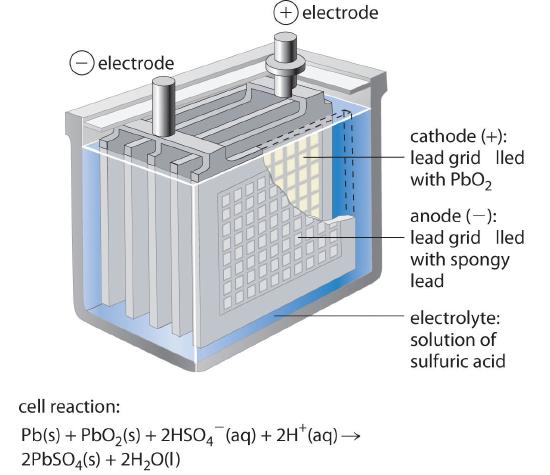
As the cell is discharged, a powder of PbSO4 forms on the electrodes. Moreover, sulfuric acid is consumed and water is produced, decreasing the density of the electrolyte and providing a convenient way of monitoring the status of a battery by simply measuring the density of the electrolyte.

Source: Photo courtesy of Mitchclanky2008, http://www.flickr.com/photos/25597837@N05/2422765479/.
When an external voltage in excess of 2.04 V per cell is applied to a lead–acid battery, the electrode reactions reverse, and PbSO4 is converted back to metallic lead and PbO2. If the battery is recharged too vigorously, however, electrolysis of water can occur, resulting in the evolution of potentially explosive hydrogen gas. (For more information on electrolysis, see Section 19.7.) The gas bubbles formed in this way can dislodge some of the PbSO4 or PbO2 particles from the grids, allowing them to fall to the bottom of the cell, where they can build up and cause an internal short circuit. Thus the recharging process must be carefully monitored to optimize the life of the battery. With proper care, however, a lead–acid battery can be discharged and recharged thousands of times. In automobiles, the alternator supplies the electric current that causes the discharge reaction to reverse.
Fuel Cells
A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Unlike a battery, it does not store chemical or electrical energy; a fuel cell allows electrical energy to be extracted directly from a chemical reaction. In principle, this should be a more efficient process than, for example, burning the fuel to drive an internal combustion engine that turns a generator, which is typically less than 40% efficient, and in fact, the efficiency of a fuel cell is generally between 40% and 60%. Unfortunately, significant cost and reliability problems have hindered the wide-scale adoption of fuel cells. In practice, their use has been restricted to applications in which mass may be a significant cost factor, such as US manned space vehicles.
These space vehicles use a hydrogen/oxygen fuel cell that requires a continuous input of H2(g) and O2(g), as illustrated in Figure 19.5.4 . The electrode reactions are as follows:
\( cathode:\; O_{2} \left (g \right )+ 4H^{+} +4e^{-} \rightarrow 2H_{2}O\left ( g \right ) \tag{19.5.20} \)
\( anode:\; 2H_{2} \left (g \right ) \rightarrow 4H^{+} + 4e^{-} \tag{19.5.21} \)
\( overall:\; 2H_{2} \left (g \right ) + O_{2}\left ( g \right ) \rightarrow 2H_{2}O\left ( g \right ) \tag{19.5.22} \)
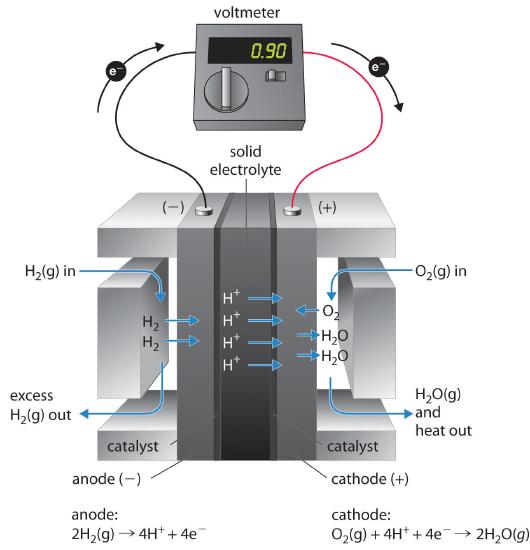
The overall reaction represents an essentially pollution-free conversion of hydrogen and oxygen to water, which in space vehicles is then collected and used. Although this type of fuel cell should produce 1.23 V under standard conditions, in practice the device achieves only about 0.9 V. One of the major barriers to achieving greater efficiency is the fact that the four-electron reduction of O2(g) at the cathode is intrinsically rather slow, which limits current that can be achieved. All major automobile manufacturers have major research programs involving fuel cells: one of the most important goals is the development of a better catalyst for the reduction of O2.
Summary
A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. One type of battery is the Leclanché dry cell, which contains an electrolyte in an acidic water-based paste. This battery is called an alkaline battery when adapted to operate under alkaline conditions. Button batteries have a high output-to-mass ratio; lithium–iodine batteries consist of a solid electrolyte; the nickel–cadmium (NiCad) battery is rechargeable; and the lead–acid battery, which is also rechargeable, does not require the electrodes to be in separate compartments. A fuel cell requires an external supply of reactants as the products of the reaction are continuously removed. In a fuel cell, energy is not stored; electrical energy is provided by a chemical reaction.
Key Takeaway
- Commercial batteries are galvanic cells that use solids or pastes as reactants to maximize the electrical output per unit mass.
Conceptual Problems
-
What advantage is there to using an alkaline battery rather than a Leclanché dry cell?
-
Why does the density of the fluid in lead–acid batteries drop when the battery is discharged?
-
What type of battery would you use for each application and why?
- powering an electric motor scooter
- a backup battery for a smartphone
- powering an iPod
-
Why are galvanic cells used as batteries and fuel cells? What is the difference between a battery and a fuel cell? What is the advantage to using highly concentrated or solid reactants in a battery?
Answer
-
- lead storage battery
- lithium–iodine battery
- NiCad, NiMH, or lithium ion battery (rechargeable)
Numerical Problem
-
This reaction is characteristic of a lead storage battery:
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)If you have a battery with an electrolyte that has a density of 1.15 g/cm3 and contains 30.0% sulfuric acid by mass, is the potential greater than or less than that of the standard cell?
Answer
-
[H2SO4] = 3.52 M; E > E°
Contributors
- Anonymous
Modified by Joshua B. Halpern


