13.6: Applications of Acid-Base Equilibria
- Page ID
- 45387
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Make sure you thoroughly understand the following essential concepts:
- Describe the major features of the global carbon cycle, and outline the role of acid-base processes in both the slow and fast parts of the cycle.
- Describe the principal acid-base reactions in aerobic respiration.
- Define acid rain, and explain its origins. Outline its major effects on the environment.
Acid-base reactions pervade every aspect of industrial-, physiological-, and environmental chemistry. In this unit we touch on a few highlights that anyone who studies or practices chemical science should be aware of.
Some applications of Buffers
Buffer solutions and the buffering effect they produce are extremely important in many practical applications of chemistry. The reason for this is that many chemical processes are quite sensitive to the pH; the extent of the reaction, its rate, and even the nature of the products can be altered if the pH is allowed to change. Such a change will tend to occur, for example, when the reaction in question, or an unrelated parallel reaction, consumes or releases hydrogen- or hydroxide ions.
Buffers in biochemistry and physiology
Many reactions that take place in living organisms fall into this category. Most biochemical processes are catalyzed by enzymes whose activities are highly dependent on the pH; if the local pH deviates too far from the optimum value, the enzyme may become permanently deactivated.
- For example, the oxidation of glucose to carbon dioxide and water — the fundamental energy-producing process of aerobic metabolism — releases H+ ions. The multiple steps of this process are catalyzed by enzymes whose activity is strongly affected by the pH. If the region of the cell where these reactions take place were not buffered, the very act of living would soon reduce the local pH below the range in which the enzymes are active, halting energy production and killing the cell.
- Blood is strongly buffered (mainly by bicarbonate) to maintain its pH at 7.4±0.3; pH values below 7.0 or above 7.8 cause death within minutes.
- The interiors of most cells are buffered near pH 7.0 (by phosphates and proteins)
Buffers in industry
Buffers are employed in a wide variety of laboratory procedures and industrial processes:
- solutions for the calibration of pH meters
- growth of bacteria in culture media
- fermentation processes, including winemaking and the brewing of beer
- controlling the colors of dyes used in coloring fabrics
- shampoos (maintaining pH at or below 7 to conteract the alkalinity of soaps that can cause irritation)
- other personal care products such as baby lotions (keeping the pH around 6 to inhibit the growth of bacteria)
- pH control in printing inks to assure their proper penetration into the paper
Buffers in the environment
- Natural waters (lakes and streams) able to support aquatic life are buffered by the sediments they are in contact with. This buffering is not always able to offset the effects of acid rain.
- Seawater is buffered mainly by bicarbonate and borates. This allows the oceans to absorb about half of the CO2 emitted to the atmosphere by human activities.
- Soils, in order to remain productive must maintain a near-neutral pH, not only for plants but also for microorganisms which fix- and recycle nitrogen. Soils rendered highly acidic by acid rain can keep essential nutrients such as phosphates in forms such as insoluble Ca3(PO4)2 that prevents their uptake by plants.
Acid-base chemistry in physiology
Acid-base chemistry plays a crucial role in physiology, both at the level of the individual cell and of the total organism. The reasons for this are twofold:
- Many of the major chemical components of an organism can themselves act as acids and/or bases. Thus proteins contain both acidic and basic groups, so that their shapes and their functional activities are highly dependent on the pH.
- Virtually all important metabolic processes involve the uptake or release of hydrogen ions. The very act of being alive tends to change the surrounding pH (usually reducing it); this will eventually kill the organism in the absence of buDering mechanisms.
About two-thirds of the weight of an adult human consists of water. About two-thirds of this water is located within cells, while the remaining third consists of extracellular water, mostly interstial fluid that bathes the cells, and the blood plasma. The latter, amounting to about 5% of body weight (about 5 L in the adult), serves as a supporting fluid for the blood cells and acts as a means of transporting chemicals between cells and the external environment. It is basically a 0.15 M solution of NaCl containing smaller amounts of other electrolytes, the most important of which are HCO3– and protein anions.
Respiration, the most important physiological activity of a cell, is an acid-producing process. Carbohydrate substances are broken down into carbon dioxide, and thus carbonic acid:
\[C(H_2O)_{n} + O_2 → CO_2 + n H_2O\]
Maintenance of acid-base balance
It is remarkable that the pH of most cellular fluids can be kept within such a narrow range, given the large number of processes that tend to upset it. This is due to the exquisite balance between a large number of interlinked processes operating at many different levels. Acid-base balance in the body is maintained by two general mechanisms: selective excretion of acids or bases, and by the buffering action of weak acid-base systems in body fluids.
- Over a 24-hour period, the adult human eliminates the equivalent of about 20-40 moles of H+ by way of the lungs in the form of CO2. In addition, the kidneys excrete perhaps 5% of this amount of acid, mostly in the form of H2PO4– and
NH4+. Owing to their electric charges, these two species are closely linked to salt balance with ions such as Na+ or K+ and Cl–. - The major buffering system in the body is the carbonate system, which exists mainly in the form of HCO3– at normal physiological pH. Secondary buffering action comes from phosphates, from proteins and other weak organic acids, and (within blood cells), the hemoglobin.
There is a huge industry aimed at the notoriously science-challenged "alternative health" market that promotes worthless nostrums such as "ionized water" that are claimed to restore or preserve "the body's acid-base balance". They commonly point out that most foods are "acidic", (i.e., they are metabolized to H2CO3), but they never explain that most of this acid is almot immediately exhaled in the form of CO2. The implication is that our health is being ruined by the resulting "acidification" of the body; some further imply this can be a cause of cancer and other assorted ailments.
People who fall for these expensive scams are in effect paying a tax on scientific ignorance. Those who, like you, have studied Chemistry, should consider that they have a social duty to counter this kind of predatory and deceptive pseudoscience.
Disturbances of acid-base balance
Deviations of the blood plasma pH from its normal value of 7.4 by more than about ±.1 can be very serious. These conditions are known medically as acidosis and alkalosis. They can be caused by metabolic disturbances such as diabetes and by kidney failure in which excretion of H2PO4– , for example, is inhibited.
Numerous other processes lead to temporary unbalances. Thus hyperventilation, which can result from emotional upset, leads to above-normal loss of CO2, and thus to alkalosis. Similarly, hypoventilation can act as a compensatory mechanism for acidosis. On the other hand, retention of CO2 caused by bronchopneumonia, for example, can give rise to acidosis. Acidosis can also result from diarrhea (loss of alkaline fluid from the intestine), while loss of gastric contents by vomiting promotes alkalosis.
Acid rain
The atmosphere is naturally acidic
The natural, unpolluted atmosphere receives acidic, basic, and neutral substances from natural sources (volcanic emissions, salt spray, windblown dust and microbial metabolism) as well as from pollution (Figure 11.6.X). These react in a kind of gigantic acid-base titration to give a solution in which hydrogen ions must predominate to maintain charge balance (indicated by the identical widths of the bar graphs at the bottom labeled "anions" and "cations").
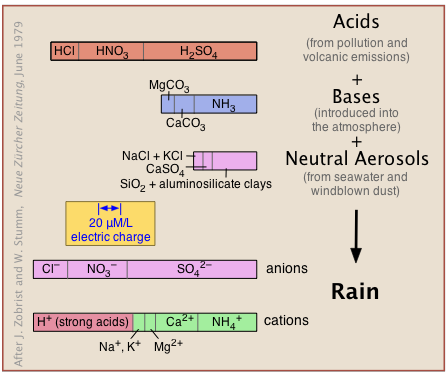
Carbon dioxide, however, is the major source of acidity in the unpolluted atmosphere. As will be explained farther on, CO2 makes up 0.032 vol-% of dry air, and dissolves in water to form carbonic acid:
\[CO_{2(g) }+ H_2O(l) \rightleftharpoons H_2CO_{3(aq)}\]
Thus all rain is “acidic” in the sense that it contains dissolved CO2 which will reduce its pH to 5.7. The term acid rain is therefore taken to mean rain whose pH is controlled by pollutants which can lower the pH into the range of 3-4, resulting in severe damage to the environment.
Origin of acid rain
Acid rain originates from emissions of SO2 and various oxides of nitrogen (known collectively as "NOx") that are formed during combustion processes, especially those associated with the burning of coal. Incineration of wastes, industrial operations such as smelting, and forest fires are other combustion-related sources; these also release particulate material into the atmosphere which plays an important role in concentrating and distributing these acid-forming substances.
Origins of NOx
Nitrogen oxides are formed naturally by soil bacteria acting on nitrate ions, an essential plant nutrient and itself a product of natural nitrogen fixation. Ammonia, a product of bacterial decomposition of organic matter, is eventually transformed into NO3– by bacteria into nitrogen oxides.
The quantity of fixed nitrogen produced industrially to support intensive agriculture now exceeds the amount formed naturally, and has become the major source of anthropogenic nitrogen oxides.
- Atmospheric nitrogen can be thermally decomposed to its various oxides NOx when subjected to high temperatures; one minor natural source is lightning. More significantly, these oxides are also formed when "clean" fuels such as natural gas (mainly methane, CH4) is burnt in air.
- NOx can also react with other atmospheric pollutants to produce photochemical smog.
Formation of acids
The gases noted above react with atmospheric oxygen, with each other, and with particulate materials to form sulfuric, nitric, and hydrochloric acids.
- Most of the H2SO4 comes from the photooxidation of SO2 released from the burning of fossil fuels and from industrial operations such as smelting.
- Nitric acid similarly results from photooxidation of nitrogen oxides. Large quantities of these oxides are formed in high-temperature combustion processes in gasoline- and jet engines, and in the turbine engines of fossil-fueled electric power plants.
- Hydrochloric acid is formed naturally through the reaction of sea salt aerosols with atmospheric H2SO4 and HNO3. The salt particles form when ocean waves break the surface and some of the water evaporates before the spray settles back to the surface. On a much smaller scale, fossil fuel combustion and waste incineration are believed to the principal human-caused sources of HCl.
Wet and dry deposition
There are two basic mechanisms by which acidic material is transported through the environment. The acid that dissolves in the water droplets that form clouds and precipitation, and is eventually incorporated into fog and snowflakes, undergoes wet deposition; this is ordinarily what is referred to as "acid rain". Because it is widely dispersed, often high in the atmosphere, it can travel very large distances from the original source.
About 40% of the acidic substances introduced into the atmosphere becomes adsorbed onto particulate matter, which can be soot, smoke, windblown dust, and salt particles formed naturally from sea spray. This spreads less widely (typically around 30 km from the source), and falls onto surfaces by "dry deposition". Once the acid-laden particles land on surfaces, ordinary rain, fog, and dew release the acidic components, often in considerably more concentrated form than occurs through dry deposition alone.
Effects on soils
Because soils support the growth of plants and of the soil microorganisms that are essential agents in the recycling of dead plant materials, acid rain has a indirect but profound effect on soil health and plant growth.
Soils containing alkaline components (most commonly limestone CaCO3 and other insoluble carbonates) can neutralize added acid and mitigate its effects. But soils in high mountain regions tend to be thin and unable to provide adequate buffering capacity. The same is true in almost half of Canada, in which granite rock of the Canadian Shield is very close to the surface; the eastern provinces of the country are strongly impacted by acid rain.
- Most soils also contain clay and humic substances that bind and retain ions such as Ca2+, Mg2+ and K+ which are essential plant nutrients. Added H+ binds even more strongly to these substances, displacing the nutrient ions from the upper layers. The overall effect is to leach these ions to greater depths where they may be inaccessible to plants, or to wash them away into ground water.
- The SO42– component of acid rain converts some nutrient cations into insoluble sulfates, reducing their availability to plants.
- Clays, which are complex aluminosilicates, gradually break down, releasing aluminum ions which normally form insoluble Al(OH)3. Addition of H+ dissolves this hydroxide, raising the concentration of Al(H2O)63+ to levels that can be toxic to plants.
- Many toxic heavy metals such as Pb, Cd and Cr are present in trace amounts in soils but are tied up in the form of insoluble salts. Acid rain can mobilize the ions of these metals, much as happens with aluminum.
Effects on plants
↓ Click on image to enlarge
Leaf damaged by acid rain
↓ Click on image to enlarge
Forest in the U.S Andirondacks damaged by acid rain
The effects on soils noted above affect plants most strongly. However, direct impingment of acidic rain and fog on leaves has other effects which can be especially serious when air pollutants such as SO2 are present.
- Acid deposition on leaves and needles tends to weaken them by eroding away their protective waxy coatings. This often results in the development of brown spots that interfere with photosynthesis.
- Forests in mountain regions are frequently bathed in clouds and fog which can be even more acidic than normal precipitation, exacerbating the above problem. In addition, the leaves and needles tend to accumulate the fog aerosol into larger droplets. These eventually fall to the forest floor, magnifying the effects on soils described in the previous section.
- Food crops tend to be less affected by acid rain where good farming practices such as the addition of fertilizers to replace depleted nutrients and the addition of limestone to raise the soil pH.
Effects on lakes and aquatic ecosystems
Lakes are subject not only to wet and dry acidic deposition, but also by the water they receive from streams and surface runoff. Any toxic elements released by the action of acid rain on soils and sediments can thus be conveyed to, and concentrated within lakes and the streams that empty them.
↓ Click on image to enlarge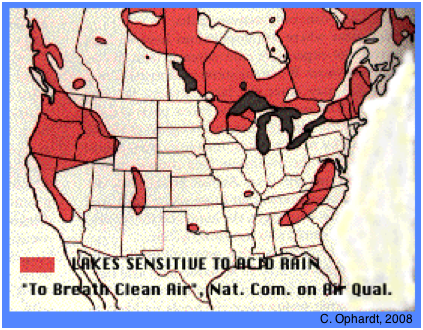
Regions of poorly-buffered lakes in North America in 1980
↓ Click on image to enlarge
May Lake in the California Sierras - a typical alpine lake
Lakes in poorly-buffered areas such as are found in alpine regions (western North America, Colorado and much of Switzerland) or on the Canadian Shield and in the Adirondacs and Appalachians) are highly sensitive to acid deposition.
Severely acidified lakes (such as the one depicted at the above right) can be so devoid of life, including algae, that the water appears to be perfectly clear and bright blue in color.
↓ Click on image to enlarge
pH tolerances of some aquatic organisms
Aquatic organisms are generally adapted to "ordinary" pH conditions of 6-8, but vary greatly in their tolerance of low pH. As pH declines below 6, the diversity of aquatic animals, plants, and microorganisms diminishes.
- Some plant species such as sphagnum mosses and certain filamentous algaes that thrive at very low pH can proliferate in acidified lakes, producing thick mats that seal off oxygen and thus inhibit the decay of litter on the lake floor.
- Acid deposited onto winter snow is released during spring melting and can cause rapid drops of pH in poorly-buffered lakes that receive waters from these sources. This "spring acid shock" can seriously affect viability of organisms such as fish, amphibians, and insects that lay their eggs in the water which hatch in the spring. The hatchlings are often unable to adapt to the rapid change, and end up deformed or killed.
-
Even those aquatic species able to survive at in low-pH waters can be indirectly affected if their food supply is pH-limited. For example, mayflies and some other insects, which, which are important food sources for frogs, cannot survive below pH 5.5.
↓ Click on image to enlarge

Fish damaged by acid deposition
- Fish are severely affected by the aluminum that is released from the action of acid on sediments; it causes a coating of mucus to form on the gills, impeding absorption of oxygen. This has led to the extinction of some species from affected lakes.
- Low pH increases the solubility of calcium salts, making it more difficult for bone to form in developing embryos of fish and amphibians.
- Low pH can also make the eggs of aquatic organism thicker and more difficult for embryos to penetrate, thus delaying hatching. When the embryos continue to grow within the confined space of the egg, their spines can be deformed, interfering with their viability when they finally hatch.
Effects on buildings and statues
Acid rain is not new!
"It has often been observed that the stones and bricks of buildings, especially under projecting parts, crumble more readily in large towns where coal is burnt... I was led to attribute this effect to the slow but constant action of acid rain." Robert Angus Smith, 1856
Acid deposition most strongly affects heritage buildings made of limestone and similar carbonate-containing stone materials.
The principal chemical process involves the reaction of sulfuric acid with calcium carbonate:
\[\ce{CO3(s) + H2SO4 → CaSO4(s) + CO2}.\]
The acid first erodes and breaks up the surface of the stone. As the hydrated calcium sulfate (gypsum) forms, it picks up iron and other components of the stone and forms an unsightly black coating. Some of this gradually blisters off, exposing yet more stone suface. The gypsum crystals can sometimes grow into the stone, further undermining the surface.
Very old structures such as the Taj Mahal, Notre Dame, the Colosseum and Westminster Abbey have all been affected.
↓ Click on image to enlarge
Stone buildings and monuments are rapidly damaged by acid rain

The exposed upper survaces of the gargoyle are beginning to deteriorate.
Statues and monuments, including those made from marble, are also susceptible to erosion and damage from acid deposition.
Modern buildings are less affected, although acid rain can erode some painted surfaces.
The Geochemical Carbonate system
The carbonate system, consisting as it does of a soluble gas CO2, soluble ions HCO3– and CO32–, and sparingly soluble carbonate salts, spans all four realms of nature: the atmosphere, hydrosphere (mainly the oceans), the lithosphere, and the biosphere. And owing to the acid-base equilibria that govern the transformations between these carbonate species, carbon is readily transported between these geochemical reservoirs. This chapter presents an overview of carbon geochemistry.
Distribution of carbon on Earth
Carbon is the fourth most abundant element in the universe. Within the Earth's crust it ranks 15th, mostly in the form of carbonates in limestones and dolomites. Kerogens, which are fossilized plant-derived carbon mostly in the form of oil shale, constitute another major repository of terrestrial carbon.
| Source | relative to atmosphere | |
|---|---|---|
|
carbonate rock
|
carbonate rock
|
28,500
|
|
fossil carbon
|
572
|
10,600
|
|
land - organic carbon
|
0.065
|
1.22
|
|
ocean
|
3.2
|
61.8
|
|
atmospheric CO2
|
0.0535
|
1.0
|
The geochemical carbon cycle
The carbonate system encompasses virtually all of the environmental compartments– the atmosphere, hydrosphere, biosphere, and major parts of the lithosphere. The complementary processes of photosynthesis and respiration drive a global cycle in which carbon passes slowly between the atmosphere and the lithosphere, and more rapidly between the atmosphere and the oceans. Thus the "carbon cycle" can be divided into "fast" and "slow" parts, operating roughly on annual and geological time scales.
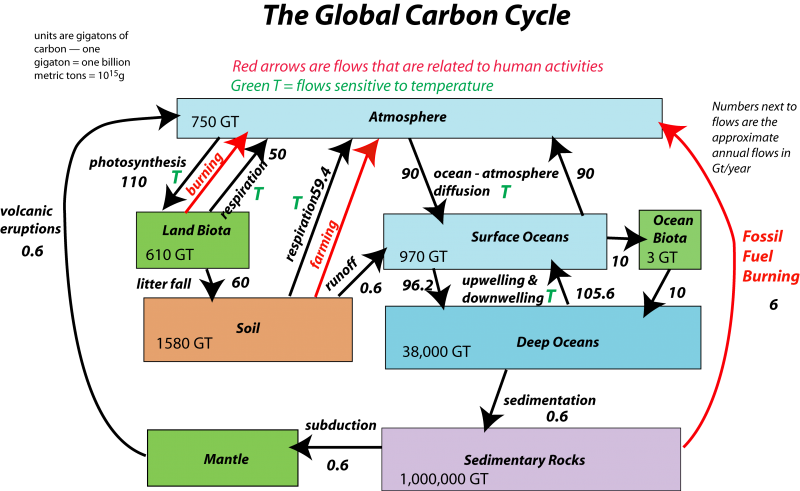
Figure (\PageIndex{2}\): Image by David Bise, Pennsylvania State University; see here for a full discussion of this diagram.
Another excellent depiction of the carbon cycle, by the U.S. Dept of Energy. (See description):
Carbon Cycle
Carbon dioxide in the atmosphere
CO2 has probably always been present in the atmosphere in the relatively small absolute amounts now observed. Precambrian limestones, possibly formed by reactions with rock-forming silicates, e.g.
\[\ce{CaSiO_3 + CO_2 → CaCO_3 + SiO_2} \label{4-1}\]
have likely had a moderating influence on the CO2 abundance throughout geological time.
The volume-percent of CO2 in dry air is 0.032%, leading to a partial pressure of 3 × 10–4 (103.5) atm. In a crowded and poorly-ventilated room, PCO2 can be as high as 100 × 10–4 atm. About 54 × 1014 moles per year of CO2 is taken from the atmosphere by photosynthesis, divided about equally between land and sea. Of this, all except 0.05% is returned by respiration (mostly by microorganisms); the remainder leaks into the slow, sedimentary part of the geochemical cycle where it can remain for thousands to millions of years. Trends in the growth of atmospheric CO2 for the past five years can be seen on this US NOAA page.
Since the advent of large-scale industrialization around 1860, the amount of CO2 in the atmosphere has been increasing. Most of this has been due to fossil-fuel combustion; in 1966, about 3.6 × 1015 g of C was released to the atmosphere; this is about 12 times greater than the estimated natural removal of carbon into sediments. The large-scale destruction of tropical forests, which has accelerated greatly in recent years, is believed to exacerbate this effect by removing a temporary sink for CO2.
About 30-50% of the CO2 released into the atmosphere by combustion remains there; the remainder enters the hydrosphere and biosphere. The oceans have a large absorptive capacity for CO2 by virtue of its transformation into bicarbonate and carbonate in a slightly alkaline aqueous medium, and they contain about 60 times as much inorganic carbon as is in the atmosphere. However, effcient transfer takes place only into the topmost
(100 m) wind-mixed layer, which contains only about one atmosphere equivalent of CO2; mixing time into the deeper parts of the ocean is of the order of 1000 years. For this reason, only about ten percent of added CO2 is taken up by the oceans.
Most of the carbon in the oceans is in the form of bicarbonate, as would be expected from the pH which ranges between 7.8 and 8.2. In addition to atmospheric CO2, there is a carbonate input to the ocean from streams. This is mostly in the form of HCO3– which derives from the weathering of rocks and terrestrial carbonate sediments, and the acid-base reaction
\[H_2CO_3 + CO_3^{2–} → 2 HCO_3^– \label{4-2}\]
which can be considered to be the source of bicarbonate in seawater. In this sense, the ocean can be considered the site of a gigantic acid-base titration in which atmospheric acids (mainly CO2 but also SO2, HCl, and other acids of volcanic origin) react with bases that originate from rocks and are introduced through carbonate-bearing streams or in windblown dust.
Carbon in the hydrosphere
Dissolution of CO2 in water
Carbon dioxide is slightly soluble in water:
| °C |
0
|
4
|
10
|
20
|
|---|---|---|---|---|
| mol L–1 |
0.077
|
0.066
|
0.054
|
0.039
|
Henry's law is followed up to a CO2 pressure of about 5 atm:
\[[\ce{CO2 (aq)}] = 0.032 P_{\ce{CO_2}} \label{4-3}\]
“Dissolved carbon dioxide” consists mostly of the hydrated oxide CO2(aq) together with a small proportion of carbonic acid:
\[[\ce{CO2 (aq)}] = 650 [\ce{H2CO3}] \label{4-4}\]
The acid dissociation constant \(K_{a1}\) that is commonly quoted for "H2CO3" is really a composite equilibrium constant that includes the above equilibrium. This means that "pure" H2CO3 (which cannot be isolated) is a considerably stronger acid than is usually appreciated.
Distribution of carbonate species in aqueous solutions
Water exposed to the atmosphere with PCO2 = 103.5 atm will take up carbon dioxide, which becomes distributed between the three carbonate species CO2, HCO3– and CO32– in proportions that depend on K1 and K2 and on the pH. The "total dissolved carbon" is given by the mass balance
\[C_t = [H_2CO_3] + [HCO_3^–] + [CO_3^{2–}] \label{4-5}\]
The distribution of these species as a function of pH can best be seen by constructing a log C-pH diagram for Ct = 10–5 M.
This Sillén plot is drawn for two different concentrations of the carbonate system. The lower one, in heavier lines, is for a 10–5 M solution, corresponding roughly to atmospheric CO2 in equilibrium with pure water. The upper plot, for a 10–3 M solution, is representative of many natural waters such as lakes and streams where the water is in contact with sediments.
It is important to note that this diagram applies only to a system in which Ct is constant. In a solution that is open to the atmosphere, this will not be the case at high pH values where the concentration of CO2 is appreciable. Under these conditions this ion will react with H2CO3 and the solution will absorb CO2 from the atmosphere, eventually resulting in the formation of a solid carbonate precipitate.
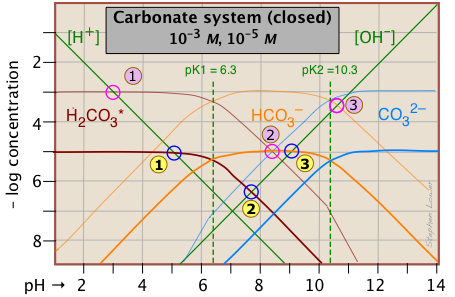
The lower of the two above plots can be used to predict the pH of 10^{5} M solutions of carbon dioxide, sodium bicarbonate, and sodium carbonate in pure water. The pH values are estimated by using the mass and charge balance conditions for each solution as noted below.
The reasoning leading to these calculations is explained in the discussion of the 10–3 M carbonate system in a previous chapter.
- Solution of CO2 or H2CO3
- [H+] = [OH–] + [HCO3–] + 2 [CO32–](4-6)
- which, since the solution will be acidic, can be simplified to
- [H+] = [HCO3–] (point
)(4-7)
- Solution of NaHCO3
- [H+] + [H2CO3] = [CO32–] + [OH](4-8)
- or
- [H+] = [HCO3–] (point
) (4-9)
- Solution of Na2CO3
- [H+] + 2 [H2CO3] + [CO32–] = [OH–](4-10)
- or
- [HCO3–] = [OH–] (point
) (4-11)
Carbon in the oceans
Natural waters aquire carbon from sediments they are in contact with, and of course also from the atmosphere. The pH is an important factor here; CO2 and solid carbonates are more soluble at high pH, and pH also controls the distribution of carbon species, as is seen in the plot just above.
| rain | river/lake water | sea water | |
|---|---|---|---|
| ppm of carbon |
1.2
|
35
|
140
|
| pH (unpolluted) |
5.6
|
6.5 - 8.5
|
7.5 - 8.4
|
At the slightly alkaline pH of most bodies of water (of which the oceans constitute 97% of the earth's surface waters), bicarbonate is the principal dissolved carbon species. The quantity of organic carbon is fairly small.
| CO2(aq) | HCO3– | CO32– | dead org. | living org. |
|---|---|---|---|---|
|
0.18
|
2.6
|
0.33
|
0.23
|
0.05
|


