10.7: Acid-Base Gallery
- Page ID
- 3484
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Make sure you thoroughly understand the following essential ideas which have been presented above. It is especially important that you know the precise meanings of all the highlighted terms in the context of this topic.
- Describe some of the special properties of sulfuric acid that make it especially important both in the laboratory and in industry.
- Name the major acids and bases that are important to the fertilizer industry
- Name the natural sources of any three of the major organic acids.
- What are fatty acids? In what major way do the physical properties of saturated and unsaturated fatty acids differ?
- Describe the general structure of an amino acid, and state why they are important.
Acids and bases are of interest not only to the chemically inclined; they play a major role in our modern industrial society — so anyone who participates in it, or who is interested in its history and development, needs to know something about them. Five of the major acids and bases fall into the "Top 20" industrial chemicals manufactured in the world. The following table shows year-2000 figures for the U.S:
| cal and rank |
Sulfuric acid - 1 |
Lime (CaO) |
Phosphoric acid - 4 |
Ammonia |
Sodium hydroxide - 9 |
Nitric acid - 11 |
|---|---|---|---|---|---|---|
| production in 109kg | 40 | 20 | 16 | 15 | 11 | 8 |
| major use | chemicals | cement | fertilizers | fertilizers | chemicals | chemicals |
The mineral acids
This term refers to any inorganic acid, but its common use is usually limited to the major strong acids plus phosphoric acid. The major mineral acids— sulfuric, nitric, and hydrochloric— have been known since medieval times. Their discovery is usually credited to the Persian alchemist Abu Musa Jabir ibn Hayyan, known in the West by his Latinized name Geber. Jabir also invented aqua regia, the mixture of nitric and hydrochloric acids that has the unique ability to dissolve gold.
Sulfuric acid
More sulfuric acid is manufactured than any other industrial chemical, and it is the cheapest industrial acid worldwide. It has been continuously manufactured in the U.S. since 1793 and in Europe for much longer.

What you should know about it
- Pure anhydrous H2SO4 is a dense, viscous liquid which melts at 10.4°C and boils at about 300°C, decomposing back into its constituents, H2O and SO3.
- The acid undergoes autoprotolysis
2 H2SO4 → H3SO4+ + HSO4–
H2SO4 + 2 NaCl → 2 Na+ + SO42– + HCl(g)
C12H22O11(s) → 12 C(s) + 11 H2O
H2SO4 + H2SO4 → HS2O7– + H3O+
- Its high boiling point makes the acid ideal for making other acids, such as nitric and hydrochloric, which are more volatile; removal of the gaseous product drives the reaction to the right as predicted by the Le Chatelier Principle:
- Sulfuric acid has a voracious appetite for water, and thus is an excellent dehydrating agent. This is seen most spectacularly if some concentrated acid is poured onto a small pile of table sugar; after a short time, a vigorous reaction ensues resulting in a pile of porous, steaming carbon
- Sulfuric acid can even dehydrate itself! , some of which have been detected on the surface of Jupiter's moon Europa.
- Owing to the autoprotolysis and self-dehydration reactions described above, "pure" sulfuric acid contains at least six minority species in addition to H2SO4.

How it is made
Sulfur trioxide, the anhydride of sulfuric acid, is the immediate precursor. Gaseous SO3 reacts vigorously with water, liberating much heat in the process:
\[SO_{3(g)} + H_2O_{(l)} → H_2SO_{4(l)}\]
Industrial manufacture of the acid starts with sulfur dioxide, prepared from burning elemental sulfur or obtained as a byproduct from roasting sulfide ores. The oxidation of SO2 to SO3 looks simple
\[SO_{2(g)} + ½ O_{2(g)} → SO_{3(g)}\]
but there are several complications:
- All chemical reactions take place more rapidly at higher temperatures, but because this reaction is highly exothermic, raising the temperature decreases the yield.
- Because of the decrease in volume (1.5 moles of gases to 1 mole), raising the pressure will increase the yield, so the reaction is carried out at a temperature below 600°C but at very high pressure.
- Specialized catalysts are used to speed up the reaction at these lower temperatures.
- Dissolving the SO3 directly in water would release large amounts of heat, creating a mist of fine acid droplets that would escape into the atmosphere. The SO3 is instead dissolved in sulfuric acid to form pyrosulfuric acid or oleum, sometimes known as fuming sufuric acid:
\[H_2SO_{4(l)} + SO_{3(g)} → H_2S_2O_{7(l)}\]
\[H_2S_2O_{7(l)} + H_2O_{(l)} → 2 H_2SO_{4(l)}\]
- The oleum is then treated with water to form industrial grade (96-98%) sulfuric acid:
What it is used for
Sulfuric acid has a broad spectrum of industrial uses, and the annual tonnage follows the economic cycle quite closely.
- Sixty percent of worldwide product goes into the manufacture of phosphoric acid H3PO4 which is used to make phosphate fertilizers and phosphate-based household detergents.
- An important nitrogen fertilizer, ammonium sulfate (NH4)2SO4, is made by reacting sulfuric acid with ammonia, the latter often obtained from the thermal decomposition of coal.
- Sulfuric acid is the major component of pickling acid that is used to remove surface oxide scale from steel before it is fabricated into steel product for the automobile and other industries.
- Aluminum sulfate (made from bauxite Al2O3 with H2SO4), is widely used in the papermaking industry to coagulate the cellulose fibers, producing a smooth, hard paper surface. Another major use is to make aluminum hydroxide which is used to filter out particulate matter in water treatment facilities.
- The familiar lead-acid storage battery employs sulfuric acid as an electrolyte. As the battery discharges, the concentraton of sulfuric acid in the electrolyte decreases as sulfate ions are taken up as PbSO4. Owing to the high density of the acid, the state of charge of the battery can be measured by means of a hydrometer.
Sulfuric acid in the environment
Acid Rain - Combustion of fossil fuels which contain organic sulfur compounds releases SO2 into the atmosphere. Photochemical oxidation of this compound to SO3, which rapidly takes up moisture, leads to the formation of H2SO4, a major component of acid rain.
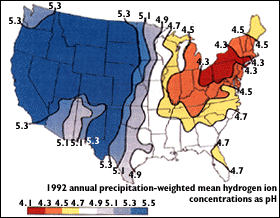
Acid mine drainage results when sediments of the very common iron pyrite, FeS2, are exposed to air and are oxidized:
FeS2(s) + 7/2 O2 + H2O → Fe2+ 2 SO42– + 2 H+
further oxidation of the iron to Fe3+ results in additional reactions. The resulting drainage liquid is often orange-brown in color and can a have a pH of below zero.
Nitric acid
Anhydrous HNO3 is a colorless liquid boiling at 82.6°C, but "pure" HNO3 only exists as the solid which melts at –41.6°C. In its liquid and gaseous states, the acid is always partially decomposed into nitrogen dioxide:
2 HNO3 → 2 NO2 + ½ O2 + H2O
This reaction, which is catalyzed by light, accounts for the brownish color of HNO3 solutions.
What you should know about it
- HNO2 undergoes autoprotolysis to a greater extent than any other liquid. Further reactions of the conjugate acid H2NO3+ with HNO3 lead to a complicated mixture of species in the liquid.
- Dilute nitric acid can be concentrated by distillation up to a maximum of 68%, at which point it forms a constant-boiling (azeotropic) mixture with water. Higher concentrations require dehydration with sulfuric acid; the result is fuming nitric acid.
- "Concentrated nitric acid" is sold as a 70% solution in water, corresponding to a concentration of about 16M.
- Nitric acid is a very strong oxidizing agent, which adds to its corrosive behavior with organic materials including, of course, skin, which it turns yellow owing to a reaction with the protein keratin. Reactions with many organic compounds are highly exothermic and often violent. The well-known reaction of nitric acid with metallic copper produces copious amounts of brown nitrogen dioxide gas.

How it is made
The simplest method, which was used industrially before 1900, was by treatment of sodium nitrate ("Chile saltpeter", NaNO3) with sulfuric acid. The direct synthesis of the acid from atmospheric nitrogen and oxygen is thermodynamically favorable
½ N2 + 5/4 O2 + ½ H2O → HNO3
but is kinetically hindered by an extremely high activation energy, a fact for which we can be most thankful (see sidebar.) The first industrial nitrogen fixation process, developed in 1903, used this reaction to produce nitric acid, but it required the use of an electric arc to supply the activation energy and was therefore too energy-intensive to be economical.
Note
If it were not for the high activation energy required to sustain this reaction, all of the oxygen in the atmosphere would be consumed and the oceans would be a dilute solution of nitric acid.
The modern Ostwald process involves the catalytic oxidation of ammonia to nitric oxide NO, which is oxidized in a further step to NO2; reaction of the latter with water yields HNO3. This route, first developed in 1901, did not become practical until the large-scale production of ammonia by the Haber-Bosch process in 1910.
What it is used for
The major industrial uses of nitric acid are for the production of ammonium nitrate fertilizer, and in the manufacture of explosives. On a much small scale, the acid is used in metal pickling, etching semiconductors and electronic circuit boards, and in the manufacture of acrylic fibers.
In the laboratory, the acid finds use in a wide variety of roles.
In the environment
High-temperature combustion processes (in internal combustion engines, power plants, and incinerators) can oxidize atmospheric nitrogen to nitric oxide (NO) and other oxides ("NOx"); the NO is then photooxidized to NO2, which reacts with water to form HNO3 which is a major component of acid rain. NO2is the major precursor of photochemical smog.
Hydrochloric acid
Unlike the other major acids, there is no such substance as "pure" hydrochloric acid; what we call "hydrochloric acid" is just an aqueous solution of hydrogen chloride gas (bp –84°C). But in a sense it is more "pure" than the acids discussed above, since there is no autoprotolysis; hydronium and chloride ions are the only significant species in the solution. Hydrochloric acid is usually sold as a 32-38% (12M) solution of HCl in water; concentrations greater than this are known as fuming hydrochloric acid.
Note
Hydrochloric acid is still sometimes sold under its older name muriatic acid for cleaning bricks and other household purposes. The name comes from the same root as marine, reflecting its preparation from salt.
The acid has been known to chemists (and alchemists), and used for industrial purposes since the middle ages. Its composition HCl was demonstrated by Humphrey Davy in 1816.
What you should know about it
- Hydrochloric acid is the least hazardous of the strong mineral acids to work with because unlike the other ones, it is not an oxidizing agent. It is usually the acid of choice for titrations and other operations in which the main requirement is simply a strong source of hydronium ions.
- The concentrated acid boils at 48°C. As boiling continues, it loses HCl and the boiling point rises to 109°C, at which point a constant-boiling (azeotropic) solution remains, consisting of 20.2% HCl.

What it is used for
The uses of hydrochloric acid are far too many to enumerate individually, but the following stand out:
- A major industrial use is to remove surface scale from iron or steel ("pickling") before it is processed into sheets or other forms, or galvanized or coated.
- Production of chlorinated organic chemicals, particularly vinyl chloride, polyurethanes, and other construction polymers, consumes huge amounts of HCl.
- The acid is widely used for pH control of water, including neutralization of wastwater streams, and for regenerating ion-exchange water softeners.
How it is made
The ancient method of treating salt with sulfuric acid to release HCl has long since been supplanted by more efficient processes, including direct synthesis by "burning" hydrogen gas in chlorine:
\[H_{2(g)} + Cl_{2(g)} \rightarrow 2 HCl_{(g)}\]
Most hydrochloric acid production now comes from reclaiming byproduct hydrogen chloride gas from other processes, especially those associated with the production of industrial organic compounds.
2 The Alkali Metals
The term alkali usually means a basic salt of a Group 1 or 2 ("alkali" or "alkaline earth") metal. All alkalies are of course bases, but the latter term is much more general, whether defined according to the Arrhenius, Brønsted-Lowry, or Lewis concepts. The word alkali comes from the Arabic al-qali, which refers to the ashes from which sodium and potassium hydroxides (potash, "ashes remaining in the pot", and the origin of the element name potassium) were extracted as a step in the making of soap.
Sodium hydroxide
Pure sodium hydroxide is a white solid consisting of Na+ and OH– ions in a crystal lattice. Although it is widely thought of as an ionic solid, van der Waals forces make a substantial contribution to its stability.
What you should know about it
- Owing to its deliquescence (ability to take up moisture) and its tendency to react with carbon dioxide, the solid must be stored in a closed container.
- In industry, sodium hydroxide is commonly known as caustic soda or simply as caustic; NaOH sold for household purposes is usually known as lye.
- Sodium hydroxide slowly attacks glass to form sodium silicate. Glass vessels used to store concentrated solutions gradually develop a cloudy coating on the inside.
- Some metals, notably aluminum, zinc, and titanium, react with strongly alkaline solutions, but iron and copper are immune to this kind of attack.
- Highly alkaline solutions also soften and dissolve skin, accounting for the slippery feeling associated with strong bases. Sodium hydroxide was once used to dispose of animal carcasses, digesting them into an easily disposable liquid form.
How it is made
Sodium hydroxide is now manufactured by the electrolysis of brine solutions, and along with chlorine, is one of the two major products of the chloralkali industry.
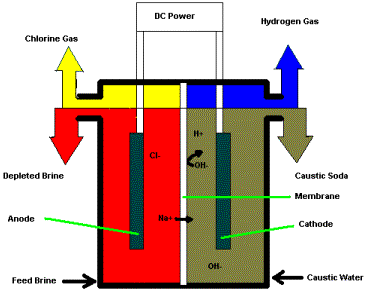
Electrolysis of aqueous NaCl produces Cl2 at the anode, but because H2O can be reduced more readily than Na+, the water is decomposed to H2 and OH– at the cathode, leaving a solution of NaOH. An older mercury cell process reduces the Na+ to Na within a mercury amalgam (alloy), and the metallic sodium is then combined with water to produce NaOH and hydrogen. The net reaction for the reduction step is the same for both methods:
\[2 Na^+ + 2 H_2O + 2e^– \rightarrow H_{2(g)] + 2 NaOH\]
The resulting solution is usually evaporated to such a high concentration that it solidifies at ordinary temperatures. It is commonly shipped in rail cars or barges which can be heated with steam to liquefy the mixture for removal. (It is obviously uneconomical to ship large quantities of water across the country!)
What it is used for
- Sodium hydroxide is one of the most diverse industrial chemicals in terms of its applications. Most householders know it as the active ingredient of drain cleaning agents.
- Huge quantities are consumed by the pulp and paper industry, which is probably its single largest specific industrial application. It is used to remove the lignin component of wood pulp from the cellulose so that the latter can be processed into paper.
- About half of the NaOH output goes into the production of a wide variety of other industrial chemicals, and in degreasing steel drums and other industrial surfaces.
- "Lye" plays a role in the processing of many types of foods, including chocolate, olives, pretzels, and the "hominy" and "grits" corn products used in the Southern U.S. It also acts as a chemical peeling agent for fruits and vegetables.

The economic push and pull of caustic and chlorine
In contrast to the extremely diverse applications of sodium hydroxide which makes the demand for this commodity relatively immune to the ups and downs of the economic cycle, the consumption of chlorine is directly dependent on the economy as reflected in the demand for polyvinyl chloride products that are now widely used in the the construction and home furnishings industries. Because chlorine, being a gas, is expensive to store, the output of the chloralkali industry is governed largely by the demand for this commodity. When times are good this presents no problem; caustic is then largely a by-product and can easily be stockpiled if supply exceeds demand. But during an economic downturn, the demand for chlorine declines, limiting its production along with that of caustic. But because the demand for caustic tends to decline much less, it becomes scarce and its price rises, thus tending to drive the industrial economy into even deeper trouble.
Sodium carbonate
This compound is known industrially as soda ash, and domestically as washing soda. The common form is the heptahydrate, Na2CO3·7 H2O. The white crystals of this substance spontaneously lose water (effloresce) when exposed to the air, forming the monohydrate.
What it is used for
- Although carbonates are much weaker bases than hydroxides, a solution of sodium carbonate can still have a pH of 11 or so, sufficiently high to allow it to substitute for sodium hydroxide in many applications— especially when the price of caustic is high.
- The single most important use of soda ash is in the manufacture of glass, where it serves to lower the melting point of the principal component, SiO2.
- Another emerging major use is to neutralize the SO2 emissions of fossil fuel-burning power plants.
- The older use of sodium carbonate as a cleaning agent (hence the name washing soda) was based partly on the ability of its alkaline solutions to emulsify grease, but mainly as a means of precipitating the insoluble carbonates of calcium and magnesium before these ions (commonly present in hard water) could form undesirable preciptates with soaps. The use of modern detergents has largely eliminated this once important market.

How it is made
Most of the world's sodium carbonate is made by the ammonia-soda "Solvay" process developed in 1861 by the Belgian chemist Ernest Solvay (1838-1922) whose patents made him into a major industrialist and a rich philanthropist. This process involves a set of simple reactions that essentially converts limestone (CaCO3), ammonia NH3 and brine (NaCl) into sodium bicarbonate NaHCO3 and eventually Na2CO3, recycling several of the intermediate products in an ingenious way.

A minor source of soda ash (but quite significant in some countries, such as the U.S.) is the mining of natural evaporites (the remains of ancient lakes), such as the trona found in Southern California.
Ammonia
Ammonia NH3 is of course not a true alkali, but it is conveniently included in this section for discussion purposes. Most people are familiar with the pungent odor of this gas, which can be detected at concentrations as low as 20-50 ppm.
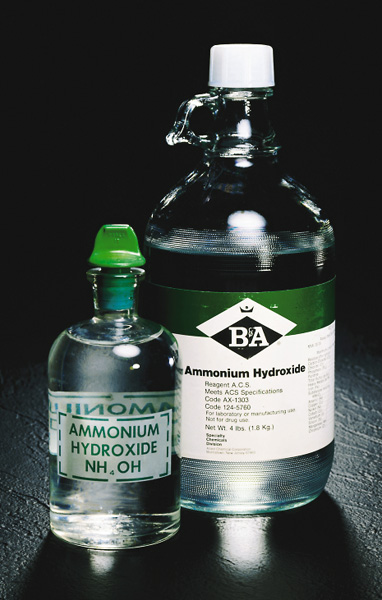
Tradition dies slowly: a non-existent chemical available in bottles!
What you should know about it
- More moles of ammonia are manufactured than of any other industrial chemical.
- Ammonia is extremely soluble in water. An aqueous solution of ammonia is still sometimes referred to in commerce as "ammonium hydroxide", but this term is no longer favored by chemists because no such compound as NH4OH has ever been shown to exist. At neutral pH, about 99% of the ammonia in water exists as NH4+ ions.
- Ammonia is an end product of nitrogen metabolism in most organisms. One source that may be familiar to parents of infants is the bacterial decomposition of the contents of diapers.
- Liquid ammonia (bp –33°C) is often used as an ionizing laboratory solvent.
What it is used for
- The major use of ammonia (about 80%) is as a fertilizer, most commonly as anhydrous ammonia (the gas is injected directly into the soil) or after conversion to (NH4)2SO4, NH4NO3, or urea O–C(NH3)2.
- Ammonia is used in the production of numerous polymers, including nylons and polyurethanes.
- Explosives manufacture accounts for about 5% of ammonia production.
- Beyond these, there are hundreds of minor uses, including as a household cleaning agent (aqua ammonia). a refrigerant, and as a laboratory reagent.
How it is made
Ammonia is made by direct synthesis from the elements:
\[N_{2(g)} + 3 H_{2(g)} \rightarrow 2 NH_{3(g)}\]
... a simple-looking reaction, but one that required some very creative work to implement; the Haber-Bosch process is considered to be the most important chemical synthesis of the 20th Century.
3 Some important organic acids
Most acids are organic— there are millions of them. The acidic function is usually a hydroxyl group connected to a carbon that is bonded to an electron-withdrawing oxygen atom; the combination is the well-known carboxyl group, –COOH. Here are a few that are part of everyone's life.
Acetic acid
This is next to formic acid in being the simplest of the organic acids, and in the form of vinegar (a 5-8% solution in water) its characteristic odor is known to everyone. The pure acid is a colorless liquid above 16.7°C; below this temperature it forms a crystalline solid, hence the term "glacial acetic acid" that is commonly applied to the pure substance. The name of the acid comes from acetum, the Latin word for vinegar.

What you should know about it
- The pure acid, although quite weak in the proton-donor sense, is quite corrosive and its vapors are very irritating.
- A 1.0M solution of the acid has a pH of about 2.4, corresponding to only four out of every thousand CH3COOH molecules being dissociated.
What it is used for
Slightly less than half of the world production of acetic acid goes into the production of polymers. The end product visible to most people would be the flexible plastic bottles in which drinking water is sold. Other uses are related mostly to the production of other chemicals, mainly acetic anhydride, but also including aspirin.

How it is made
Bacterial fermentation of sugars has been the source of vinegar since ancient times, and is still accounts for most food-grade acetic acid and vinegar, but it now amounts to only about 10% of total acetic acid production:
C6H12O6 → 3 CH3COOH
There are several important synthetic routes to acetic acid production, but the major one is by treating methanol with carbon monoxide:
CH3OH + CO → CH3COOH



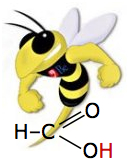 Formic acid
Formic acid Oxalic acid
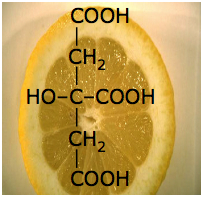
Oxalic acid
 No carboxyl groups here, but the –OH group one carbon away from the double bond is still fairly acidic. One of its geometric isomers, L-ascorbic acid, is more widely known as Vitamin C; discovery of the essential role of this substance in preventing the disease scurvy (from which its name is derived) yielded two Nobel prizes in 1937. Most animals are capable of synthesizing their own Vitamin C, but primates (along with guinea pigs) seem to have lost the required gene somewhere along the way, so we must depend on fruits and veggies for our supply. (Because ascorbic acid is soluble in water, it tends to be leached out of vegetables when they are boiled, so it is much healthier to steam them.)
No carboxyl groups here, but the –OH group one carbon away from the double bond is still fairly acidic. One of its geometric isomers, L-ascorbic acid, is more widely known as Vitamin C; discovery of the essential role of this substance in preventing the disease scurvy (from which its name is derived) yielded two Nobel prizes in 1937. Most animals are capable of synthesizing their own Vitamin C, but primates (along with guinea pigs) seem to have lost the required gene somewhere along the way, so we must depend on fruits and veggies for our supply. (Because ascorbic acid is soluble in water, it tends to be leached out of vegetables when they are boiled, so it is much healthier to steam them.)
 The acid itself turned out to be a bit too much of an irritant to the stomach's lining, so the German firm Bayer began marketing a tamed version, acetylsalicylic acid (ASA) under the name Aspirin in 1899, and it has been going strong ever since. Interestingly, the detailed chemistry of its pain-and-fever reliving action was not discovered until 1970.
The acid itself turned out to be a bit too much of an irritant to the stomach's lining, so the German firm Bayer began marketing a tamed version, acetylsalicylic acid (ASA) under the name Aspirin in 1899, and it has been going strong ever since. Interestingly, the detailed chemistry of its pain-and-fever reliving action was not discovered until 1970.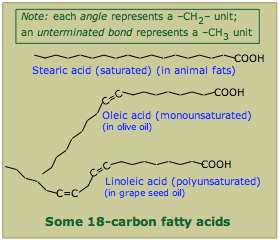
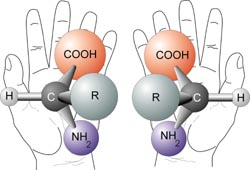
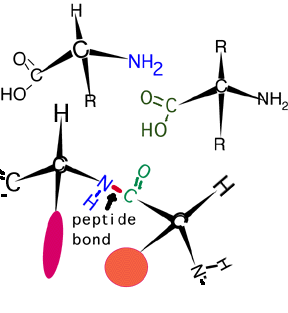 The carboxylic acid part of one amino acid can react with the amine part of another to form a peptide bond (an amide linkage) shown here.
The carboxylic acid part of one amino acid can react with the amine part of another to form a peptide bond (an amide linkage) shown here.