Water Chemistry
- Page ID
- 37362
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Water is an unusual compound with unique physical properties. As a result, its the compound of life. Yet, its the most abundant compound in the biosphere of Earth. These properties are related to its electronic structure, bonding, and chemistry. However, due to its affinity for a variety of substances, ordinary water contains other substances. Few of us has used, seen or tested pure water, based on which we discuss its chemistry.
The chemistry of water deals with the fundamental chemical property and information about water. Water chemistry is discussed in the following subtitles.
- Composition of water
- Structure and bonding of water
- Molecular Vibration of water
- Symmetry of water molecules
- Formation of hydrogen bonding in water
- Structure of ice
- Autoionization
- Leveling effect of water and acid-base characters
- Amphiprotic nature
- Reactivity of water towards alkali metals; alkaline earth metals; halogens; hydrides; methane; oxides; and oxygen ions.
- Electrolysis of water
Composition of Water
Water consists of only hydrogen and oxygen. Both elements have natural stable and radioactive isotopes. Due to these isotopes, water molecules of masses roughly 18 (H216O) to 22 (D218O) are expected to form. Isotopes and their abundances of H and O are given below. From these data, we can estimate the relative abundances of all isotopic water molecules.
| 1H | 2D | 3T | ||
|---|---|---|---|---|
| 99.985% | 0.015% | 12.33 y | ||
| 14O | 15O | 16O | 17O | 18O |
| 70.6 s | 122 s | 99.762% | 0.038% | 0.200% |
The predominant water molecules H216O have a mass of 18 amu, but molecules with mass 19 and 20 occur significantly. Because the isotopic abundances are not always the same due to their astronomical origin, The isotopic distribution of water molecules depends on its source and age. Its study is linked to other sciences. (See Dojlido, J.R. & Best, G.A. (1993) Chemistry of Water and Water Pollution, Ellis Harwood for isotopic distribution of water.)
| H216O | H218O | H217O | HD16O | D216O | HT16O |
| 99.78% | 0.20% | 0.03% | 0.0149% | 0.022 ppm | trace |
| 18 | 20 | 19 | 19 | 20 | 20 amu |
In particular, D216O is called heavy water, and it is produced by enrichment from natural water. Properties of heavy water are particularly interesting due to its application in nuclear technology.
Structure and Bonding of the Water Molecule
Pure water, H2O, has a unique molecular structure. The O-H bondlengths are 0.096 nm and the H-O-H angle = 104.5°. This strange geometry can be explained by various methods. From carbon to neon, the numbers of valence electrons increase from 4 to 8. These elements require 4, 3, 2, 1, and 0 H atoms to share electrons in order to complete the octet requirement. Their Lewis dot structures are shown on the right, and note the trend in bondlengths.
There are six valance electrons on the oxygen, and one each from the hydrogen atom in the water molecule. The eight electrons form two H-O bonds, and left two lone pairs. The long pairs and bonds stay away from each other and they extend towards the corners of a tetrahedron. Such an ideal structure should give H-O-H bond angle of 109.5°, but the lone pairs repel each other more than they repel the O-H bonds. Thus, the O-H bonds are pushed closer, making the H-O-H angle less than 109°.
After the introduction of quantum mechanics, the electronic configuration for the valence electron of oxygen are 2s2 2p4. Since the energy levels of 2s and 2p are close, valence electrons have characters of both s and p. The mixture is called sp3 hybridization. These hybridized orbitals are shown on the right. The structures of CH4, NH3, and H2O can all explained by these hybrid orbitals of the central atoms. The above approach is the valence bond theory, and both the C-H bonds and lone electron pairs are counted as VSPER pairs in the Valence-shell Electron-Pair Repulsion (VSEPR) model, according to which, the four groups point to the corners of a tetrahedron.
For triatomic molecules such as water, molecular orbital (MO) approach can also be applied to discuss the bonding. The result however is similar to the valence bond approach, but the MO theory gives the energy levels of the electron for further exploration.
Molecular Vibration of Water
Atoms in a molecule are never at rest, and for each type of molecule, there are some normal vibration modes. For the water molecule, the three normal modes of vibrations are symmetric stretching, bending and asymmetric stretching. The vibrations are quantized, as do any microscopic system, and their quantum numbers are designated as v1, v2 and v3. The observed transition bands of D2O, H2O, and HDO are given in the table below.
| Quantum numbers of upper state | Absorption wavenumbers of bands /cm-1 | ||||
|---|---|---|---|---|---|
| v1 | v2 | v3 | D2O | H2O | HDO |
| 0 | 1 | 0 | 1178 | 1594 | 1402 |
| 1 | 0 | 0 | 2671 | 3656 | 2726 |
| 0 | 0 | 1 | 2788 | 3756 | 3703 |
| 0 | 1 | 1 | 3956 | 5332 | 5089 |
| Data from Eisenberg, D. and Kauzmann, W. (1969) Structure and properties of water, Oxford University press. | |||||
The ideal transition bands are centered in the given wavenumbers. However, these wavenumbers are calculated based on isolated molecules with no interaction with any neighbor. When molecules interact with each other, the energy levels are modified, and the bands shift.
Many more less intense absorption bands extend into the green part of the visible spectrum. The absorption spectrum of water may contribute to the blue color for lake, river and ocean waters.
Symmetry of Water Molecules
The water molecules are rather symmetric in that there are two mirror planes of symmetry, one containing all three atoms and one perpendicular to the plane passing through the bisector of the H-O-H angle. Furthermore, if the molecules are rotated 180° (360°/2) the shape of the molecule is unperturbed. This indicates that the molecules have a 2-fold rotation axis. The three symmetry elements are 2-fold rotation, and two mirror planes. Both mirror planes contain the rotation axis, and this type of symmetry belongs to the point group C2v. A point group has a definite number of symmetry elements arranged in certain fashion. Molecules can be classified according to their point groups. Molecules of the same point group have similar spectroscopic characters. Other molecules of C2v point group are CH2=O, CH2Cl2, the bent O3 etc.
Formation of Hydrogen Bonding
Under certain conditions, an atom of hydrogen is attracted by rather strong forces to two atoms instead of only one, so that it may be considered to be acting as a bond between them. This is called hydrogen bond. This statement is from Linus Pauling (1939) in his book The Nature of the Chemical Bond. He gave the ion [F:H:F]- as an example. At that time, the hydrogen bond was recognized as mainly ionic in nature. The energy associated with hydrogen bond is 8 to 40 kJ/mol.
| Molecule | Molar mass |
m.p. | b.p. /° C |
|---|---|---|---|
| NH3 | 17 | -77.8 | -33.5 |
| H2O | 18 | -0 | 100 |
| H2S | 34 | -85.6 | -60 |
| H2Se | 81 | -60.4 | -41.5 |
| H2Te | 128.6 | -51 | -1.8 |
| CH3OH | 32 | ? | 65 |
| C2H5OH | 46 | ? | 78 |
| C2H5OC2H5 | 74 | ? | 34 |
Normally, the melting point and boiling point of a substance increase with molecular mass. For example the melting points of inert gases are 0.95, 24.48, 83.8, and 116.6 K respectively for He, Ne, Ar, and Kr.
In this table, the melting and boiling points for water are particular high for its small molecular mass. This is usually attributed to the formation of hydrogen bonds. The small electronegative atoms F, O and N are somewhat negatively charged when they are bonded to hydrogen atoms. The negative charges on F, O and N attract the slightly positive hydrogen atoms, forming a strong interaction called hydrogen bond.
A graph showing the melting points and boiling points of group 16 provided by Prof. J. Boucher illustrates the same point.
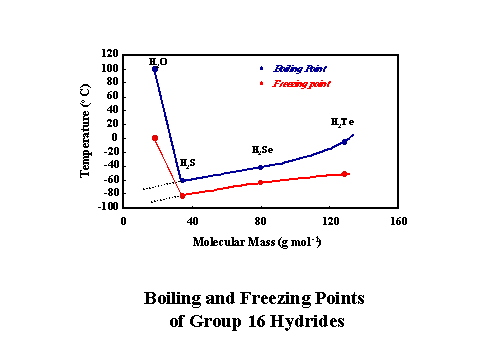
Based on the observed absorption at 3546 and 3691 cm-1, Van Thiel, Becker, and Pinmentel (1957, J. Chem. Phys. 27 386) suggested the formation of water dimer when trapped in a matrix of nitrogen.
Due to hydrogen bonding, water molecules form dimers, trimers, polymers, and clusters. The hydrogen bonds are not necessarily liner.
Structure of Ice
Ice occurs in many places, including the Antarctic. If all the ice melted, the water level of the oceans will rise about 70 m. The structure of ice and the caption are from this link.
The density of ice is dramatically smaller than that of water, due to the regular arrangement of water molecule via hydrogen bonds. In an idealized structure of ice, every hydrogen atom is involved in hydrogen bond. Every oxygen atom is surrounded by four hydrogen bonds.
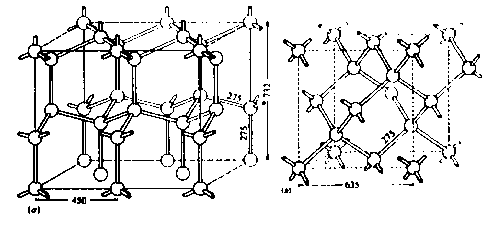
This diagram from caltech.edu, shows the structure of hexagonal ice in (a) and cubic ice in (b). A rod here represents a hydrogen bond. Since the hydrogen bonds are not linear, the real structure is a little more complicated.
The tetrahedral coordination opens up the space between molecules. On each hydrogen bond, shown by a rod joining the oxygen atoms, lies one proton in an asymmetric position (not shown). Bond lengths, 275 pm, are indicated. Ordinary ice is hexagonal. and the hexagonal c axis is labelled 732 pm, and one of the hexagonal a axes is labelled 450 pm. If water vapor condenses on very cold substrate at 143-193 K (-130 to -80ºC) a cubic phase is formed. In (b) the cubic unit cell is outlined with dashed lines; dimensions are in pm determined at 110 K.
These diagrams can also be used to represent the two forms of diamond, and in this case, the rods joining the atoms represent C-C bonds. Each C-C bondlength is 154 pm. Silicon and germanium crystals have the same structure, but their bondlengths are longer. The two diamond types of structure are related to the packing of spheres. The hexagonal type has the ABABAB... sequence, whereas the cubic type has the ABCABC... sequence. In both cases, half of the tetrahedral sites are occupied by tetrahedrally bonded carbon atoms. Hexagonal diamonds have been observed in meteorites.
 The four hydrogen bonds around an oxygen atom form a tetrahedron in a fashion found in the two types of diamonds. Thus, ice, diamond, and close packing of spheres are somewhat topologically related.
The four hydrogen bonds around an oxygen atom form a tetrahedron in a fashion found in the two types of diamonds. Thus, ice, diamond, and close packing of spheres are somewhat topologically related.
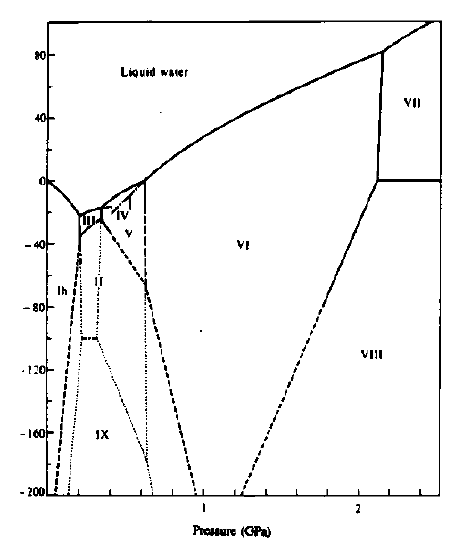
A phase diagram of water shows 9 different solid phases (ices). Ice Ih is the ordinary ice. In addition to ice Ic from vapor deposition, conditions for nine phases are shown. Aside from ice I, other phases are formed and observed under high pressure generated by machines built by scientists. So far, ten different forms of ice have been observed, and some ice forms exist at very high pressure. The pressure deep under the polar (Antarctic) ice cap is very high, but we are not able to make any direct observation or study.
There is a report of the 11th ice, and the ice phase diagram and drawings of ice structures given here is extremely interesting.
The Autoionization of Water
The Autoionization of Water in the formation of ions according to
HOH(l) + HOH(l) = H3O+ + OH-
This is an equilibrium process and is characterised by an equilibrium constant, K'w:
[H3O+] [OH-]
K'w = ------------
[H2O]
Since [H2O] = 1000/18 = 55.56 M, and remains rather constant under any circumstance, we usually write
Kw = [H3O+] [OH-]
= 10-14 (or 1e-14)
pKw = -log Kw (defined)
= 14 (at 298 K)
| tºC | Kw |
|---|---|
| 20 | 1.14e-15 |
| 25 | 1.00e-14 |
| 35 | 2.09e-14 |
| 40 | 2.92e-14 |
| 50 | 5.47e-14 |
For neutral water, [H3O+] = [OH-] = 1e-7 at this temperature. Furthermore, we define
pH = -log[H3O+]
pOH = -log[OH-]
pH = pOH = 7 at 298 K; (in neutral solutions)
It is important to realize that Kw depends on temperature as shown in the Table here.
Leveling Effect of Water and Acid-Base Characters
The strength of strong acids and bases is dominated by the autoionization of water. In aqueous solutions, the strongest acid and base are the hydronium ion, H3O+, and the hydroxide ion OH- respectively. Acids HCl, HBr, HI, HNO3, HClO3, HClO4, and H2SO4 completely ionize in water, making them as strong as H3O+ due to the leveling effect of water. Furthermore, strong acids, strong bases, and salts completely ionize in their aqueous solutions.
For example, HCl is a stronger acid than H2O, and the reaction takes place as HCl dissolves in water.
HCl + H2O = Cl- + H3O+
A similar equation can be written for another strong acid.
On the other hand, a stong base also react with water to give the stong base species, OH-.
H2O + B- = OH- + HB
For example, O2-, CH3O-, and NH3 are strong bases. The leveling effect also apply to bases.
Amphiprotic Species
Equilibria of acids and bases, are interesting chemistry. When an acid and a base differ by a proton, they are called a conjugate acid-base pair. A water molecule is a weak acid and base, due to its ability to accept or donate a proton. Such properties make water an amphiprotic species. In fact, H3O+, H2O and OH- are amphiprotic, as are some other conjugate acid-base pairs of weak acids and bases.
If several acids and bases are dissolved in water, all equilibria must be considered. To estimate the pH of these solutions requires the exact treatment of several equilibrium constants. For example, many species dissolve in rain water, and many equilibria must be considered. Detail consideration and examples are given in Acid-Base Reactions
Carbon dioxide in the air dissolve in rain water, lakes and rivers. A solution of CO2 involves the following reaction:
| Reaction | K formula | K value |
|---|---|---|
| H2O(l) + CO2(g) = H2CO3(l) | 1/PCO2 | ? |
| H2CO3 = HCO3- + H+ | [HCO3-] [H+] / [H2CO3] | 5e-7 |
| HCO3- = CO3-2 + H+ | [CO3-2] [H+] / [HCO3-] | 5e-11 |
| HOH(l) + HOH(l) = H3O+ + OH- | [H3O+] [OH-] | 1e-14 |
These complicated equilibria make natural water a buffer.
Example 1
Assume that the partial pressure of carbon dioxide causes a total concentration of carbonic species to be 8e-4 M. Estimate the pH of this solution.
Solution
From the given data, we have the following five equations and five unknowns:
| Equilibrium | Equations | No. |
|---|---|---|
| H2CO3 « HCO3- + H+ | [HCO3-] [H+] ---------------- = 5e-7 [H2CO3] |
(1) |
| HCO3- « CO32- + H+ | [CO32-] [H+] -------------- = 5e-11 HCO3- |
(2) |
| 2 H2O « H3O+ + OH- | [H3O+] [OH-] = 1e-14 | (3) |
| Charge balance | [H+] = [HCO3-] + [OH-] + 2 [CO32-] |
(4) |
| All species containing C | [H2CO3] + [HCO3-] + [CO32-] = 8.0e-4 M |
(5) |
| Unknowns | ||
| [H+], [OH-], [H2CO3], [HCO3-], [CO32-] | ||
Solving these equations for the 5 unknowns can be done using Maple, Mathcad, spread sheet, or approximation. In any case, we are interested in the pH, and we can make the following approximations or assumptions
| Assume H+ mostly comes from (1) |
[H+] = [HCO3-] | |
| H2CO3 is a weak acid most unionize |
[H2CO3] = 8.0e-4 M | (6) |
| Let x = [HCO3-] = [H+] | [HCO3-] [H+] / [H2CO3] = x2 / [H2CO3] = 5.0e-7 |
(1) |
Combining (1) and (6) gives [H+]2 = x2 = 8.0e-4 * 5.0e-7 = 4.0e-10. Therefore,
[H+] = 2.0e-5
pH = -log(2.0e5) = 4.7
DISCUSSION
Generally speaking, rain water has a pH about 5, rather acidic. It dissolves limestone and marble readily. Due to the dissolved carbon dioxide, rain water is a buffer solution.
Increased carbon dioxide level forces an increase in dissolved carbon dioxide. Would this causes pH of rain water to decrease or increase? Justify your answer by giving the reasons.
Since [H+] = 2.0e-5, [OH-] = 5e-9, the amount of H+ from ionization of water is also 5.0e-9, small with respect to 2.0e-5 from ionization of H2CO3. Similarly, the ionization from
HCO3- « CO32- + H+
is also small. Most of the C-containing species is H2CO3
H2CO3 is a weak acid, its ionization is small indeed.
Now, you may proceed to evaluate other concentrations: [OH-], [HCO3-], and [CO32-]
Reactivity of Water Towards Metals
Alkali metals react with water readily. Contact of cesium metal with water causes immediate explosion, and the reactions become slower for potassium, sodium and lithium. Reaction with barium, strontium, calcium are less well known, but they do react readily. Warm water may be needed to react with calcium metal, however. Many metals displace H+ ions in acidic solutions. This is often seen as a property of acids.
Electrolysis of Water
The enthalpy of formation for liquid water, H2O(l), is -285.830 and that of water vapour is -241.826 kJ/mol. The difference is the heat of vaporization at 298 K. Liquid water and vapor entropies (S) are 69.95 and 188.835 kJ K-1 mol-1 respectively, (see Thermodynamic Data. These are entropies, not standard entropies of formation. The entropy of formation for water is obtained by,
DSof water = Sowater - SoH2 - 0.5 SoO2
= 69.95 - 130.68 - 0.5*205.14 (data from Thermodynamic Data)
= - 163.3 J K-1 mol-1
DGowater = DH - T DS (note H in kJ/mol and S in J/mol)
DGowater = -285.83 - 298.15 * 163.3/1000 = -237.13 kJ
The equilibrium constant and Gibb's energy are related,
DGo = - R T ln K
K = exp(- DGo / R T)
= 3.5e41 atm-3/2
This is a very large value for the formation of water,
H2 + 0.5 O2 = 0.5 H2O(l).
In other words, the reaction is complete, and the possibility of water dissociated into hydrogen and oxygen is very small. A negative value for DGo indicates an exothermic reaction.
The Gibb's energy is the energy released other than pressure-volume work. This redox reaction to form water can be engineered to proceed in a Daniel cell. In this case, the energy is converted into electric energy according to this equation.
DGowater = - n F E = -237.13 kJ
where n is the number of electrons (= 2) in the redox equation, F is the Faraday constant (= 96485 C), and E is the potential of the Daniel cell. Thus,
- 237130 J
E = - -------------
2*96485 C
= 1.23 V
Ideally, a reverse voltage of 1.23 V is required for the electrolysis of water. But in reality, a little over voltage is required to carry out the electrolysis to decompose water. Furthermore, pure water does not conduct electricity, and acid, base or salt is often added for the electrolysis of water. This link has a demonstration.
Example 2
In order to carry out the electrolysis of water, 1.50 V is applied. Assume the energy not converted to chemical energy is converted to heat. How much heat is generated for the electrolysis of 1 mole water?
Solution
Ideally, 1.23 V will be used for the electrolysis. Energy due to the over voltage of 1.50 - 1.23 = 0.27 V is converted to heat.
Heat = 0.27 V * 2 * 96485 C
= 52102 J
= 52 kJ
DISCUSSION
The excess energy can also be evaluated using
Heat = n F *1.50 - 237130
This problem also illustrates the principle of conservation of energy.
Questions
- For the reaction
H2O(l) -> H2(g) + 0.5 OH2(g)
the equilibrium constant as shown earlier is 1/3.5e41 = 2.9e-43 atm3/2. What is the partial pressure of H2(g)?
Solutions
-
Skill -
Evaluate this value please!
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)


