Natural Water
- Page ID
- 37368
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Discussion Questions
- What is hard water?
- What are the differences between temporary and permanent hard water?
- How can hard water be converted to soft water?
- How to produce deionized water?
Water is the most important resource. Without water life is not possible. From a chemical point of view, water, H2O, is a pure compound, but in reality, you seldom drink, see, touch or use pure water. Water from various sources contains dissolved gases, minerals, organic and inorganic substances.
The Hydrosphere
The total water system surrounding the planet Earth is called the hydrosphere. It includes freshwater systems, oceans, atmosphere vapour, and biological waters. The Arctic, Atlantic, Indian, and Pacific oceans cover 71% of the Earth surface, and contain 97% of all water. Less than 1% is fresh water, and 2-3 % is ice caps and glaciers. The Antarctic Ice Sheet is almost the size of North America continent. These waters dominate our weather and climate, directly and indirectly affecting our daily lives. They cover 3.35x108 km2. The four oceans have a total volume of 1.35x109 km3.
- The sunlight dims by 1/10 for every 75 m in the ocean, and humans barely see light below 500 m. The temperature of almost all of the deep ocean is 4°C (277 K).
- The average ocean depth is 4 km, and the deepest point at the Mariana Trench is 10,912 m (35,802 ft), which compares to the height of 8.8 km for Mount Everest.
Hydrospheric processes are steps by which water cycles on the planet Earth. These processes include sublimation of ice, evaporation of liquid, transportation of moisture by air, rain, snow, river, lake, and ocean currents. All these processes are related to the physical and chemical properties of water, and many government agencies are set up to study and record phenomena related to them. The study of these processes is called hydrology
Among the planets, Earth is the only one in which there are solid, liquid and gaseous waters. These conditions are just right for life, for which water is a vital part. Water is the most abundant substance in the biosphere of Earth. Groundwater is an important part of the water system. When vapor is cooled, clouds and rain develop. Some of the rain percolate through the soil and into the underlying rocks. The water in the rocks is groundwater, which moves slowly.
A body of rock, which contains appreciable quantities of water, is called an aquifier. Below the water table, the aquifier is filled (or saturated) with water. Above the water table is the unsaturated zone. Some regions have two or more water tables. These zones are usually separated by water-impermeable material such as boulder and clay. Groundwater can be brought to the surface by drilling below the water table, and pumped out. The amount of water that can be pumped out depends on the structure of the aquifer. Little water is stored in tight granite layers, but large quantities of water are stored in limestone aquifier layers. In some areas, there are under ground rivers.
| Species | \(\ce{Cl^{-}}\) | \(\ce{Na^{+}}\) | \(\ce{SO4^{^-}}\) | \(\ce{Mg^{2+}}\) | \(\ce{Ca^{2+}}\) | \(\ce{K^{+}}\) | \(\ce{HCO3^{-}}\) | \(\ce{Br^{-}}\) | \(\ce{Sr^{2+}}\) | \(\ce{BO4^{3-}}\) | \(\ce{F^{-}}\) | \(\ce{H4SiO4}\) | \(\ce{H^{+}}\) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/Kg | 10,760 | 2,710 | 2,710 | 1,290 | 411 | 399 | 142 | 67 | 8 | 4.5 | 1.3 | 0.5-10 | \(10^{-8.35}\) |
Common Ions Present in Natural Water
Hydrology is also the study of how solids and solute interact in, and with, water. In this link, the compositions of seawater, composition of the atmosphere, compositions of rain and snow, and compositions of river waters and lake waters are given in details. Table \(\PageIndex{1}\) list the major ions present in seawater. The composition does vary, depending on region, depth, latitude, and water temperature. Waters at the river mouths contain less salt. If the ions are utilized by living organism, its contents vary according to the populations of organisms.
Dust particles and ions present in the air are nucleation center of water drops. Thus, waters from rain and snow also contain such ions: Ca2+, Mg2+, Na+, K+, NH4+. These cations are balanced by anions, HCO3-, SO4-, NO2-, Cl-, and NO3-. The pH of rain is between 5.5 and 5.6. Rain and snow waters eventually become river or lake waters. When the rain or snow waters fall, they interact with vegetation, top soil, bed rock, river bed and lake bed, dissolving whatever is soluble. Bacteria, algae, and water insects also thrive. Solubilities of inorganic salts are governed by the kinetics and equilibria of dissolution. The most common ions in lake and river waters are the same as those present in rainwater, but at higher concentrations. The pH of these waters depends on the river bed and lake bed. Natural waters contain dissolved minerals. Waters containing Ca2+ and Mg2+ ions are usually called hard water.
Hard Water
Minerals usually dissolve in natural water bodies such as lakes, rivers, springs, and underground waterways (ground waters). Calcium carbonate, CaCO3, is one of the most common inorganic compounds in the Earth crust. It is the ingredient for both calcite and aragonite. These two minerals have different crystal structures and appearance. This photograph shows crystals of typical Calcite.
Calcium-carbonate minerals dissolve in water, with a solubility product as shown below.
\[CaCO_3 \rightleftharpoons Ca^{2+} + CO_3^{2-} \;\;\; K_{sp} = 5 \times 10^{-9}\]
From the solubility product, we can (see example 1) evaluate the molar solubility to be 7.1x10-5 M or 7.1 mg/L (7.1 ppm of CaCO3 in water). The solubility increases as the pH decrease (increase acidity). This is compounded when the water is saturated with carbon dioxide, CO2. Saturated CO2 solution contains carbonic acid, which help the dissolution due to the reaction:
\[H_2O + CO_2 \rightleftharpoons H_2CO_3\]
\[CaCO_3 + H_2CO_3 \rightleftharpoons Ca^{2+} + 2 HCO_3^-\]
Because of these reactions, some natural waters contain more than 300 ppm calcium carbonates or its equivalents.
The carbon dioxide in natural water creates an interesting phenomenon. Rainwater is saturated with CO2, and it dissolves limestones. When CO2 is lost due to temperature changes or escaping from water drops, the reverse reaction takes place. The solid formed, however, may be a less stable phase called aragonite, which has the same chemical formula as, but a different crystal structure than that of calcite.
The rain dissolves calcium carbonate by the two reactions shown above. The water carries the ions with it, sips through the crack of the rocks. When it reached the ceiling of a cave, the drop dangles there for a long time before fallen. During this time, the carbon dioxide escapes and the pH of the water increases. Calcium carbonate crystals begin to appear. Calcite, aragonite, stalactite, and stalagmite are four common solids found in the formation of caves.
Natural waters contain metal ions. Water containing calcium, magnesium and their counter anions are called hard waters. Hard waters need to be treated for the following applications.
- Heat transfer carrier in boilers and in cooling systems
- Solvents and reagents in industrial chemical applications
- Domestic water for washing and cleaning
Temporary vs. Permanent Hard Water
Due to the reversibility of the reaction,
\[CaCO_{3(s)} + H_2CO_3 \rightleftharpoons Ca^{2+} + 2 HCO_3^-\]
water containing Ca2+, Mg2+ and CO32- ions is called temporary hard water, because the hardness can be removed by boiling. Boiling drives the reverse reaction, causing deposit in pipes and scales in boilers. The deposits lower the efficiency of heat transfer in boilers, and diminish flow rates of water in pipes. Thus, temporary hard water has to be softened before it enters the boiler, hot-water tank, or a cooling system. The amount of metal ions that can be removed by boiling is called temporary hardness
After boiling, metal ions remain due to presence of chloride ions, sulfate ions, nitrate ions, and a rather high solubility of MgCO3. Amount of metal ions that can not be removed by boiling is called permanent hardness. Total hardness is the sum of temporary hardness and permanent hardness. Hardness is often expressed as equivalence of amount of calcium ions in the solution. Thus, water conditioning is an important topic. The value of water treatment market has been estimated to be worth $30 billion.
Lime-soda Softening
Lime-soda softening is the removal of temporary hardness by adding a calculated amount of hydrated lime, Ca(OH)2:
\[Ca^{2+} + 2 HCO_3^- + Ca(OH)_{2(s)} \rightarrow 2 CaCO_{3(s)} + 2 H_2O\]
Adding more lime causes the pH of water to increase, and as a result, magnesium ions are removed by the reaction:
\[Mg^{2+} + Ca(OH)_{2(s)} \rightarrow Mg(OH)_{2(s)} + Ca^{2+}\]
The extra calcium ions can be removed by the addition of sodium carbonate.
\[Na_2CO_3 \rightarrow 2 Na^+ + CO_3^{2-}\]
\[Ca^{2+} + CO_3^{2-} + \rightarrow CaCO_{3(s)}\]
In this treatment, the amount of Ca(OH)3 required is equivalent to the temporary hardness plus the magnesium hardness. The amount of sodium carbonate required is equivalent to the permanent hardness. Thus, lime-soda softening is effective if both the temporary and total hardness have been determined. The sodium ion will remain in the water after the treatment. The pH of the water is also rather high depending on the amount of lime and sodium carbonates used.
Complexation Treatment
Addition of complexing reagent to form soluble complexes with Ca2+ and Mg2+ prevents the formation of solid. One of the complexing agents is sodium triphosphate Na3PO4, which is marketed as Calgon, etc. The phosphate is the complexing agent. Other complexing agents such as Na2H2EDTA can also be used, but the complexing agent EDTA4- forms strong complexes with transition metals. This causes corrosion problem, unless the pipes of the system are made of stainless steel.
Ion Exchange
Today, most water softeners are using zeolites and employing ion exchange technique to soften hard water. Zeolites are a group of hydrated crystalline aluminosilicates found in certain volcanic rocks. The tetrahedrally coordinated aluminum and silicon atoms form AlO4 and SiO4 tetrahedral groups. They interconnect to each other sharing oxygen atoms forming cage-type structures as shown on the right. This diagram and the next structural diagram are taken from an introduction to zeolites There are many kinds of zeolites, some newly synthesized.
Whatever kind, the crystal structure of zeolites contains large cages. The cages are connected to each other forming a framework with many cavities and channels. Both positive and negative ions can be trapped in these cavities and channels as shown below.
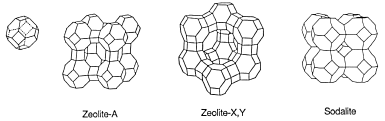
For each oxygen that is not shared in the AlO4 and SiO4 tetrahedral groups, a negative charge is left on the group. These negative charges are balanced by trapping alkali metal and alkaline earth metal ions. When more cations are trapped, hydroxide and chloride ions will remain in the cavities and channels of the zeolites.
To prepare a zeolite for water treatment, they are soaked in concentrated NaCl solution. The cavities trap as many sodium ions as they can accommodate. After the treatment, the zeolite is designated as Na-zeolite. Then the salt solution is drained, and the zeolite is washed with water to eliminate the extra salt. When hard water flow through them, calcium and magnesium ions will be trapped by the Na-zeolite. For every Ca2+ or Mg2+ trapped, two Na+ ions are released. The treated water contains a rather high concentration of Na+ ions, but low concentrations of Mg2+ and Ca2+. Thus, zeolite ion exchange convert hard water into soft water.
Pure Water by Ion Exchange
In most cases, the resins are polystyrene with functional -SO3H groups attached to the polymer chain for cation exchange resin, and with functional group -N(CH3)3+ attached to the chain for anion exchange resin. To prepare the resin for making pure or deionized water, the cation resin is regenerated with HCl so that the groups are really -SO3H. The anion resin is regenerated with NaOH, so that the functional groups are -N(CH3)3(OH). When water containing any metal ion M+ and anion A- passes through the ion exchange resins in two stages, the following reactions take place,
\[\ce{M+ + -SO3H \rightarrow H+ + -SO3M}\]
\[\ce{A^{-} + -N(CH3)3(OH) \rightarrow OH^{-} + -N(CH3)3A}\]
\[\ce{H^{+} + OH- <=> H2O}\]
Thus, ion exchange provides pure water to meet laboratory requirement.
Reverse Osmosis Water Filter System
This method can also be used to prepare water for domestic and laboratory applications. This method has been discussed in Wastewater treatment
Magnetic Water Treatment
The following is a list of companies selling magnetic devices for magnetic water treatment. All devices are based on results of some ressearch indicating that when water runs through a magnetic field, the calcium carbonate will precipitate as aragonite rather than the usual calcite. For example, K.J. Kronenberg has published an article in IEEE Transactions on Magnetics, (Vol. Mag-21, No. 5, September 1985, pages 2059-2061). and stated the following:
The crystallization mode of the water's mineral content was found to change from a dendritic, substrate-bound solidification habit to the form of separate disc-shaped crystals after the water had moved through a number of magnetic fields. The former scarcity of crystallization nucleii in the water had been turned into an abundance of nucleation centers in the water. The reduction of the number of the substrate-bound crystals has been used as a quantitative measure of the magnetic effect.
Many companies have made various devices for magnetic conditioning of water, and they claim that their devices will clean up the pipes and boilers at little or no cost. I have yet to test one of these devices for its claim, but my preliminary tests shows that permanent magnet has little effect on the calcium carbonate deposit of temporary hard water. The cleaning effect they have claimed is probably much overstated.
Example \(\PageIndex{1}\)
From the solubility product shown for the dissolution of calcium carbonate,
\[CaCO_3 \rightleftharpoons Ca^{2+} + CO_3^{2-} \;\;\; K_{sp} = 5 \times 10^{-9}\]
Evaluate the molar solubility of Ca2+ in saturated solution.
Solution
From the definition of solubility product, we have
\[[Ca^{2+}] [CO_3^{2-}] = 5 \times 10^{-9}\]
Thus,
\[[Ca^{2+}] = [CO_3^{2-}] = 7.1 \times 10^{-5}\; M\]
The concentration of 7.1x10-5 M is equivalent to 7.1 mg/L (7.1 ppm of CaCO3 in water).
DISCUSSION
There may be other ions present in the system and other equilibria conditions in addition to the equilibrium mentioned here. Problems are more complexed in the real world.
Exercise \(\PageIndex{1}\)
Boiling of 1.0 L of water produced 10 mg of CaCO3 solid. What is the temporary hardness of the water?
- Answer
-
10 ppm
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)


