3.3: Procedure, flowchart, and datasheets for separation and confirmation of group I cations
- Page ID
- 369521
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)|
Chemical |
Hazard |
|---|---|
|
0.1M Lead(II) nitrate in 0.1M nitric acid |
Toxic, irritant, and oxidant |
|
0.1M Mercury(I) nitrate in 0.1M nitric acid |
Highly toxic and oxidant |
|
0.1M silver nitrate in 0.1M nitric acid |
Toxic, corrosive, and oxidant |
- *Hazards of 6M ammonia, 6M hydrochloric acid, and 6M nitric acid are listed in a common reagents table in chapter 2.
- Used heavy metal ion solutions or precipitates are disposed of in a labeled metal waste disposal container, do not drain these solutions down the drain or in the regular trash.
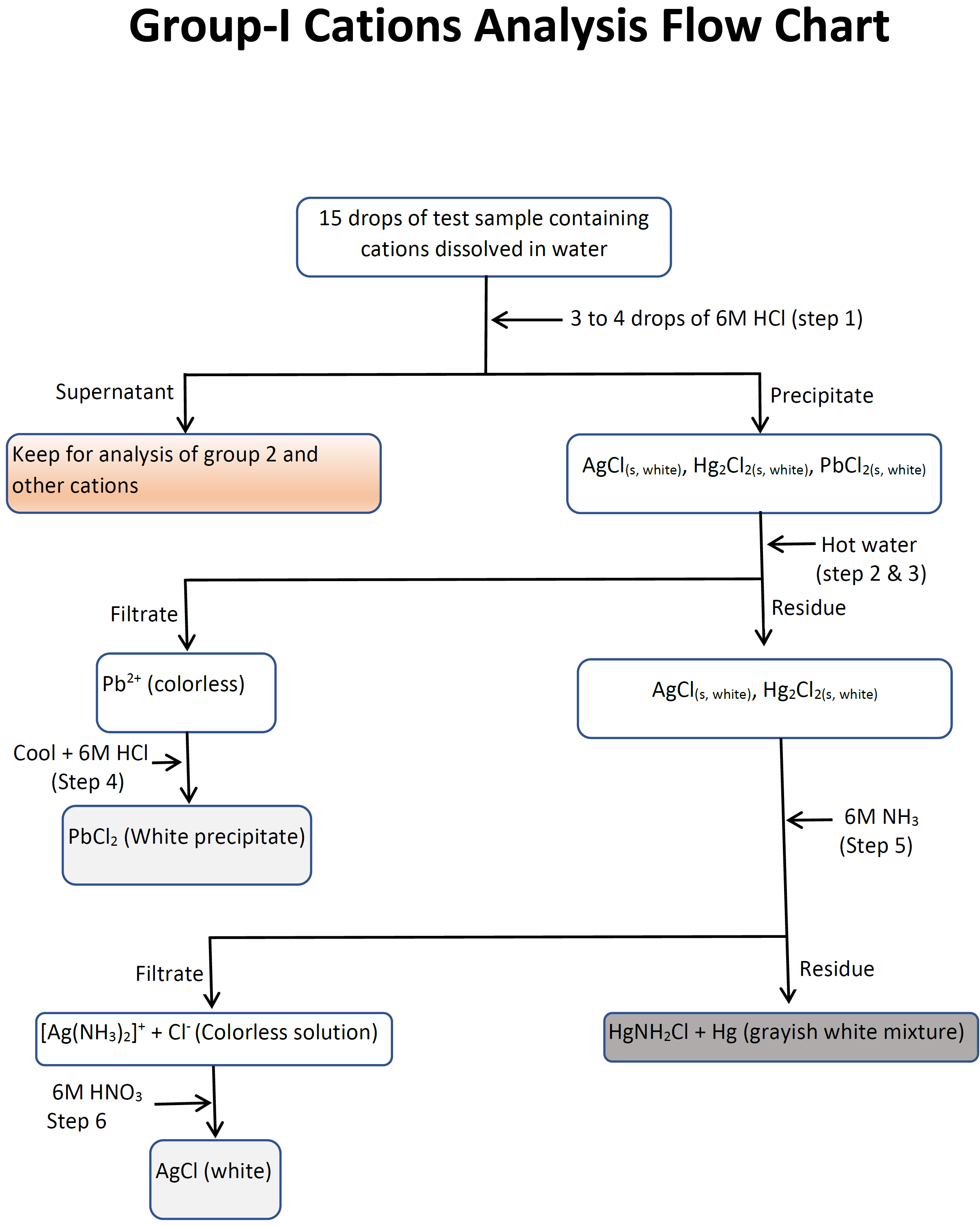
- Take 15 drops of the unknown solution in a test tube and add 3 to 4 drops of 6M \(\ce{HCl}\) to it drop by drop while stirring. Centrifuge for 2 min and without decanting add 1 more drop of 6M \(\ce{HCl}\) to check there is no more precipitation formation. If more precipitate forms, centrifuge and check again till no more precipitate forms upon addition of a drop 6M \(\ce{HCl}\) to the supernatant. Carefully decant and keep the supernatant for analysis of group 2 cations, and use the precipitate in the next step for separation and conformation group 1 cation, i.e., \(\ce{AgCl(s, white)}\), \(\ce{Hg2Cl2(s, white)}\), \(\ce{PbCl2(s, white)}\). Record the observations in the datasheet.
- Add 2 mL (40 drops) of distilled water to the precipitate from step 1 in a test tube, stir it with a clean glass rod to re-suspend the precipitate, and heat the test tube in a boiling water bath for 3 min while stirring. Add 15 mL of distilled water to a 2nd test tube and heat it also in the boiling water bath.
- Prepare a gravity filtration setup and pass ~5 mL of hot water from the 2nd test tube of step 2 to make it a heated gravity filtration setup. Discard the filtrate which is just hot water. Place an empty test tube labeled "Lead(II) confirmation test" under the heated filter funnel, filter the contents of the first test tube of step 2 and collect it and keep the filtrate for \(\ce{Pb^{2+}}\) test in the test tube labeled "Lead (II) confirmation test". Keep the residue, if there is any, for \(\ce{Ag^{+}}\) and \(\ce{Hg2^{2+}}\) tests. If there is no precipitate left, it means \(\ce{Ag^{+}}\) and \(\ce{Hg2^{2+}}\) ions were absent in the test sample.
- Let the 2 mL filtrate, in the test tube labeled "Lead (II) confirmation test", cool down to room temperature by placing the test tube in a room temperature water bath. If white crystals/precipitate forms in the filtrate upon cooling \(\ce{Pb^{2+}}\) was present in the test sample. If no crystal forms upon cooling, add 2 to 3 drops of 6M \(\ce{HCl}\) to the filtrate while stirring with a glass rod. If white crystals/precipitate forms \(\ce{Pb^{2+}}\) was present in the test sample. If no white crystals/precipitate is observed at this stage \(\ce{Pb^{2+}}\) was absent in the test sample. Discard the mixture in the metal waster container. Record the observation in the datasheet.
- Re-suspend the residue, if there is any, from step 3 in ~5 mL of hot water from the 2nd test tube of step 2 to dissolve any residual \(\ce{PbCl2}\) and then filter it out. Wash the residue with the remaining ~5 mL hot water from the 2nd test tube of step 2. Discard the filtrate which is just the wash liquid with impurities in it and leave the precipitate on the filter paper. Put a clean empty test tube under the filtration funnel and add 40 drops (2 mL) of 6M \(\ce{NH3}\) onto the residue drop by drop with genal stirring with a glass rod. Keep the filtrate for \(\ce{Ag^{+}}\) test. If residue is still left on the filter paper and changes color from white to grayish-black \(\ce{Hg2^{2+}}\) was present in the test sample, otherwise, \(\ce{Hg2^{2+}}\) was absent in the test sample. Discard the gray residue in the metal waste container. Record the observation on the datasheet.
- Add 6M \(\ce{HNO3}\) drop by drop to the 2 mL filtrate from step 5, while stirring and keep testing with blue litmus paper until the solution turns from alkaline to acidic indicated by the change of litmus paper color from blue to red. If white suspension/precipitate is observed at this stage it confirms that \(\ce{Ag^{+}}\) was present in the test sample, otherwise, \(\ce{Ag^{+}}\) was not present in the test sample. discard the mixture in the metal waste container. Record the observations in the datasheet.
- Step number refers to the corresponding step number in the procedure sub-section.
- In “the expected chemical reaction and expected observations column”, write an overall net ionic equation of the reaction that will happen if the ion being processed in the step was present, write the expected color change of the solution, the expected precipitate formed and its expected color, etc.
- In the “the actual observations and conclusion” column write the color change, the precipitate formed and its color, etc. that is actually observed as evidence, and state the specific ion as present or absent.
- In “the overall conclusion” row write one by one symbol of the ions being tested with a statement “present” or “absent” followed by evidence/s to support your conclusion.