3.2: Separation and confirmation of individual ions in group I precipitates
- Page ID
- 369497
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Separation and confirmation of lead(II) ion
Solubility of \(\ce{PbCl2}\) in water at 20 oC is about 1.1 g/100 mL, which is significantly higher than 1.9 x 10-4 g/100 mL for \(\ce{AgCl}\) and 3.2 x 10-5 g/100 mL for \(\ce{Hg2Cl2}\). Further, the solubility of \(\ce{PbCl2}\) increases three-fold to about 3.2 g/100 mL in boiling water at 100 oC, while solubility \(\ce{AgCl}\) and \(\ce{Hg2Cl2}\) remain negligible. A 15 drops sample that is used to precipitate out group I cations corresponds to about 0.75 mL, which based on the molar mass of \(\ce{PbCl2}\) is 278.1 g and the concentration of each ion ~0.1M, contains about 0.02 g of \(\ce{PbCl2}\) precipitate. This 0.02 g of \(\ce{PbCl2}\) requires ~0.6 mL of heated water for dissolution. The precipitated is re-suspended in ~2 mL water and heated in a boiling water bath to selectively dissolve \(\ce{PbCl2}\), leaving any \(\ce{AgCl}\) and \(\ce{Hg2Cl2}\) almost undissolved, as shown in Figure \(\PageIndex{1}\).
\[\ce{ PbCl2 (s) <=>[Hot~water] Pb^{2+}(aq) + 2Cl^{-}(aq)}\nonumber\]
The heated suspension is filtered using a heated gravity filtration set up to separate the residue comprising of \(\ce{AgCl}\) and \(\ce{Hg2Cl2}\) from filtrate containing dissolved \(\ce{PbCl2}\).

The solubility of \(\ce{PbCl2}\) is three times less at room temperature than in boiling water. Therefore, the 2 mL filtrate is cooled to room temperature to crystalize out \(\ce{PbCl2}\) :
\[\ce{Pb^{2+}(aq) + 2Cl^{-}(aq) <=>[Cold~water] PbCl2(s)}\nonumber\]
If \(\ce{PbCl2}\) crystals are observed in the filtrate upon cooling to room temperature, it is a confirmation of \(\ce{PbCl2}\) in the test solution. If \(\ce{PbCl2}\) concentration is low in the filtrate, the crystals may not form upon cooling. Few drops of 5M \(\ce{HCl}\) are mixed with the filtrate to force the crystal formation based on the common ion effect of Cl- in the reactants. The formation of \(\ce{PbCl2}\) crystals confirms \(\ce{Pb^{2+}}\) as shown in Figure \(\PageIndex{2}\), and no crystal formation at this stage confirms that \(\ce{Pb^{2+}}\) was absent in the test solution.

Separating mercury(I) ion from silver(I) ion and confirming mercury(I) ion
The residue left after filtering out \(\ce{Pb^{2+}}\) in hot water, is washed further with 10 mL of hot water to washout residual \(\ce{PbCl2}\). Then 2 mL of 6M aqueous \(\ce{NH3}\) solution is passed through the residue drop by drop. Aqueous \(\ce{NH3}\) dissolves \(\ce{AgCl}\) precipitate by forming water soluble complex ion \(\ce{[Ag(NH3)2(aq)]^+}\) through following series of reactions:
\[\ce{AgCl(s) <=> Ag^{+}(aq) + Cl^{-}(aq)}\quad K_f = 1.8\times10^{-10}\nonumber\]
\[\ce{Ag^{+}(aq) + 2NH3(aq) <=> Ag(NH3)2^{+}(aq)}\quad K_f = 1.7\times10^7\nonumber\]
\[\text{Overall reaction:}~\ce{AgCl(aq) + 2NH3(aq) <=> Ag(NH3)2^{+}(aq) + Cl^{-}(aq)}\quad K = 3.0\times10^{-3}\nonumber\]
The 2 mL filtrate is collected in a separate test tube for confirmation of \(\ce{Ag^+}\) ion. Although \(\ce{Hg2Cl2}\) precipitate is insoluble in water, it does slightly dissociate like all ionic compounds. The \(\ce{Hg2^{2+}}\) ions undergo auto-oxidation or disproportionation reaction producing black Hg liquid and \(\ce{Hg2^{2+}}\) ions. The \(\ce{Hg2^{2+}}\) ions react with \(\ce{NH3}\) and \(\ce{Cl^-}\) forming white water-insoluble \(\ce{HgNH2Cl}\) precipitate through the following series of reactions:
\[\ce{Hg2Cl2(s) <=> Hg2^{2+}(aq) + 2Cl^{-}(aq)}\nonumber\]
\[\ce{Hg2^{2+}(aq) <=> Hg(l) + Hg^{2+}(aq)}\nonumber\]
\[\ce{Hg^{2+}(aq) + 2NH3(aq) + Cl^{-}(aq) <=> HgNH2Cl(s) + NH4^{+}(aq)}\nonumber\]
\[\text{Overall reaction:}\ce{~Hg2Cl2(s, white) + 2NH3(aq) <=> HgNH2Cl(s, white) + NH4^{+}(aq) + Cl^{-}(aq) + Hg(l, black)}\nonumber\]
A mixture of white solid \(\ce{HgNH2Cl}\) and black liquid Hg appears gray in color. Turning of white \(\ce{Hg2Cl2}\) precipitate to grayish color upon addition of 6M \(\ce{NH3}\) solution drops confirms \(\ce{Hg2^{2+}}\) ions are present in the test solution as shown in Figure \(\PageIndex{3}\). If the white precipitate redissolves leaving behind no grayish residue, it means the precipitate was \(\ce{AgCl}\) and \(\ce{Hg2^{2+}}\) were absent in the test solution.
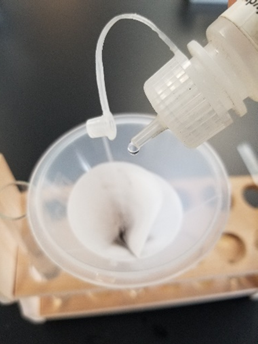
Confirming silver(I) ion
Although water-soluble complex ion \(\ce{[Ag(NH3)2(aq)]^+}\) is quite stable, it does slightly decompose into \(\ce{Ag^+}\) and \(\ce{NH3(aq)}\). The excess \(\ce{NH3}\) added to dissolve \(\ce{AgCl}\) precipitate and the that produced by dissociation of \(\ce{[Ag(NH3)2(aq)]^+}\) is removed by making the solution acidic by adding 6M \(\ce{HNO3}\). The \(\ce{Cl^-}\) formed from the dissolution of \(\ce{AgCl}\) precipitate in the earlier reactions is still present in the medium. Decomposition of \(\ce{[Ag(NH3)2(aq)]^+}\) in the acidic medium produces enough \(\ce{Ag^+}\) ions to re-form white \(\ce{AgCl}\) precipitate by the following series of equilibrium reactions.
\[\ce{[Ag(NH3)2]^{+}(aq) <=> Ag^{+}(aq) + 2NH3(aq)}\nonumber\]
\[\ce{2NH3(aq) + 2H3O^{+}(aq) <=> 2NH4^{+}(aq) + 2H2O(l)}\nonumber\]
\[\ce{Ag^{+}(aq) + Cl^{-}(aq) <=> AgCl(s, white)}\nonumber\]
\[\text{Overall reaction:}\ce{~[Ag(NH3)2]^{+}(aq) + 2H3O^{+}(aq) + Cl^{-}(aq) <=> AgCl(s, white) + 2NH4^{+}(aq) + 2H2O(l)}\nonumber\]
The formation of white \(\ce{AgCl}\) precipitate at this stage in the acidified filtrate confirms \(\ce{Ag^+}\) ion was present in the test solution, as shown in Figure \(\PageIndex{4}\), and its absence confirms that \(\ce{Ag^+}\) ion was not present in the test solution.



