23.2: Metallic Indicator Electrodes
- Page ID
- 333588
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In potentiometry, the potential of the indicator electrode is proportional to the analyte’s activity. Two classes of indicator electrodes are used to make potentiometric measurements: metallic electrodes, which are the subject of this section, and ion-selective electrodes, which are covered in the next section.
Electrodes of the First Kind
If we place a copper electrode in a solution that contains Cu2+, the electrode’s potential due to the reaction
\[\mathrm{Cu}^{2+}(a q)+2 e^{-} \rightleftharpoons \mathrm{Cu}(s) \nonumber \]
is determined by the activity of Cu2+.
\[E=E_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\mathrm{o}}-\frac{0.05916}{2} \log \frac{1}{a_{\mathrm{Cu}^{2+}}}=+0.3419 \mathrm{V}-\frac{0.05916}{2} \log \frac{1}{a_{\mathrm{Cu}^{2+}}} \nonumber \]
If copper is the indicator electrode in a potentiometric electrochemical cell that also includes a saturated calomel reference electrode
\[\mathrm{SCE} \| \mathrm{Cu}^{2+}\left(a q, a_{\mathrm{Cu^{2+}}}=x\right) | \text{Cu}(s) \nonumber \]
then we can use the cell potential to determine an unknown activity of Cu2+ in the indicator electrode’s half-cell
\[E_{\text{cell}}= E_{\text { ind }}-E_{\text {SCE }}= +0.3419 \mathrm{V}-\frac{0.05916}{2} \log \frac{1}{a_{\mathrm{Cu}^{2+}}}-0.2224 \mathrm{V} \nonumber \]
An indicator electrode in which the metal is in contact with a solution containing its ion is called an electrode of the first kind. In general, if a metal, M, is in a solution of Mn+, the cell potential is
\[E_{\mathrm{call}}=K-\frac{0.05916}{n} \log \frac{1}{a_{M^{n+}}}=K+\frac{0.05916}{n} \log a_{M^{n+}} \nonumber \]
where K is a constant that includes the standard-state potential for the Mn+/M redox couple and the potential of the reference electrode.
For a variety of reasons—including the slow kinetics of electron transfer at the metal–solution interface, the formation of metal oxides on the electrode’s surface, and interfering reactions—electrodes of the first kind are limited to the following metals: Ag, Bi, Cd, Cu, Hg, Pb, Sn, Tl, and Zn.
Many of these electrodes, such as Zn, cannot be used in acidic solutions because they are easily oxidized by H+.
\[\mathrm{Zn}(s)+2 \mathrm{H}^{+}(a q)\rightleftharpoons \text{ H}_{2}(g)+\mathrm{Zn}^{2+}(a q) \nonumber \]
Electrodes of the Second Kind
The potential of an electrode of the first kind responds to the activity of Mn+. We also can use this electrode to determine the activity of another species if it is in equilibrium with Mn+. For example, the potential of a Ag electrode in a solution of Ag+ is
\[E=0.7996 \mathrm{V}+0.05916 \log a_{\mathrm{Ag}^{+}} \label{second1} \]
If we saturate the indicator electrode’s half-cell with AgI, the solubility reaction
\[\operatorname{Agl}(s)\rightleftharpoons\operatorname{Ag}^{+}(a q)+\mathrm{I}^{-}(a q) \label{second2} \]
determines the concentration of Ag+; thus
\[a_{\mathrm{Ag}^{+}}=\frac{K_{\mathrm{sp}, \mathrm{Agl}}}{a_{\text{I}^-}} \label{second3} \]
where Ksp,AgI is the solubility product for AgI. Substituting Equation \ref{second3} into Equation \ref{second1}
\[E=0.7996 \text{ V}+0.05916 \log \frac{K_{\text{sp, Agl}}}{a_{\text{I}^-}} \label{second4} \]
shows that the potential of the silver electrode is a function of the activity of I–. If we incorporate this electrode into a potentiometric electrochemical cell with a saturated calomel electrode
\[\mathrm{SCE} \| \mathrm{AgI}(s), \text{ I}^-\left(a q, a_{\text{I}^-}=x\right) | \mathrm{Ag}(\mathrm{s}) \label{second5} \]
then the cell potential is
\[E_{\mathrm{cell}}=K-0.05916 \log a_{\text{I}^-} \label{second6} \]
where K is a constant that includes the standard-state potential for the Ag+/Ag redox couple, the solubility product for AgI, and the reference electrode’s potential.
If an electrode of the first kind responds to the activity of an ion in equilibrium with Mn+, we call it an electrode of the second kind. Two common electrodes of the second kind are the calomel and the silver/silver chloride reference electrodes.
In an electrode of the second kind we link together a redox reaction and another reaction, such as a solubility reaction. You might wonder if we can link together more than two reactions. The short answer is yes. An electrode of the third kind, for example, links together a redox reaction and two other reactions. Such electrodes are less common and we will not consider them in this text.
Metallic Redox Electrodes
An electrode of the first kind or the second kind develops a potential as the result of a redox reaction that involves the metallic electrode. An electrode also can serve as a source of electrons or as a sink for electrons in an unrelated redox reaction, in which case we call it a redox electrode. The Pt cathode in \(\PageIndex{1}\) is a redox electrode because its potential is determined by the activity of Fe2+ and Fe3+ in the indicator half-cell. Note that a redox electrode’s potential often responds to the activity of more than one ion, which limits its usefulness for direct potentiometry.
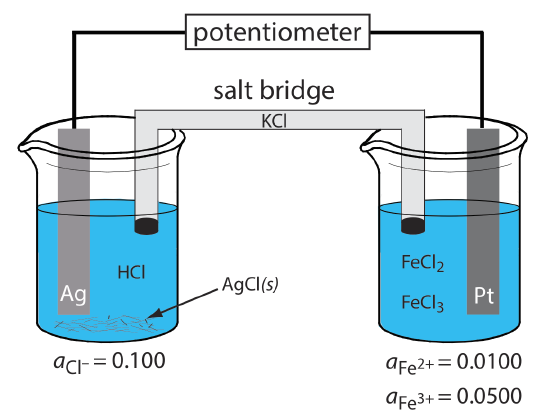
Figure \(\PageIndex{1}\). Potentiometric electrochemical cell in which the anode is a metallic electrode of the first kind (Ag) and the cathode is a metallic redox electrode (Pt).


