16.12: Formation Constants
- Page ID
- 135872
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The following table provides \(K_i\) and \(\beta_i\) values for selected metal–ligand complexes, arranged by the ligand. All values are from Martell, A. E.; Smith, R. M. Critical Stability Constants, Vols. 1–4. Plenum Press: New York, 1976. Unless otherwise stated, values are for 25 oC and zero ionic strength. Those values in brackets are considered less reliable.
|
Acetate \(\ce{CH3COO-}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Mg2+ | 1.27 | |||||
| Ca2+ | 1.18 | |||||
| Ba2+ | 1.07 | |||||
| Mn2+ | 1.40 | |||||
| Fe2+ | 1.40 | |||||
| Co2+ | 1.46 | |||||
| Ni2+ | 1.43 | |||||
| Cu2+ | 2.22 | 1.41 | ||||
| Ag+ | 0.73 | –0.09 | ||||
| Zn2+ | 1.57 | |||||
| Cd2+ | 1.93 | 1.22 | –0.89 | |||
| Pb2+ | 2.68 | 1.40 |
|
Ammonia \(\ce{NH3}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Ag+ | 3.31 | 3.91 | ||||
| Co2+ (T = 20 °C) | 1.99 | 1.51 | 0.93 | 0.64 | 0.06 | –0.73 |
| Ni2+ | 2.72 | 2.17 | 1.66 | 1.12 | 0.67 | –0.03 |
| Cu2+ | 4.04 | 3.43 | 2.80 | 1.48 | ||
| Zn2+ | 2.21 | 2.29 | 2.36 | 2.03 | ||
| Cd2+ | 2.55 | 2.01 | 1.34 | 0.84 |
|
Chloride \(\ce{Cl-}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Cu2+ | 0.40 | |||||
| Fe3+ | 1.48 | 0.65 | ||||
|
Ag+ (\(\mu = 5.0 \text{ M}\)) |
3.70 | 1.92 | 0.78 | –0.3 | ||
| Zn2+ | 0.43 | 0.18 | –0.11 | –0.3 | ||
| Cd2+ | 1.98 | 1.62 | –0.2 | –0.7 | ||
| Pb2+ | 1.59 | 0.21 | –0.1 | –0.3 |
|
Cyanide \(\ce{CN-}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Fe2+ | 35.4 (\(\beta_6\)) | |||||
| Fe3+ | 43.6 (\(\beta_6\)) | |||||
| Ag+ | 20.48 (\(\beta_2\)) | 0.92 | ||||
| Zn2+ | 11.07 (\(\beta_2\)) | 4.98 | 3.57 | |||
| Cd2+ | 6.01 | 5.11 | 4.53 | 2.27 | ||
| Hg2+ | 17.00 | 15.75 | 3.56 | 2.66 | ||
| Ni2+ | 30.22 (\(\beta_4\)) |
|
Ethylenediamine \(\ce{H2NCH2CH2NH2}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Ni2+ | 7.38 | 6.18 | 4.11 | |||
| Cu2+ | 10.48 | 9.07 | ||||
| Ag+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 4.700 | 3.00 | ||||
| Zn2+ | 5.66 | 4.98 | 3.25 | |||
| Cd2+ | 5.41 | 4.50 | 2.78 |
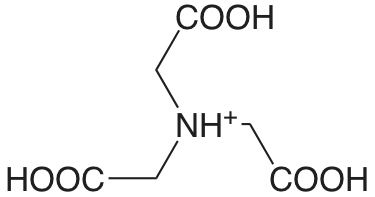
|
EDTA
|
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Mg2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 8.79 | |||||
| Ca2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 10.69 | |||||
| Ba2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 7.86 | |||||
| Bi3+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 27.8 | |||||
| Co2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 16.31 | |||||
| Ni2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 18.62 | |||||
| Cu2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 18.80 | |||||
| Cr3+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | [23.4] | |||||
| Fe3+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 25.1 | |||||
| Ag+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 7.32 | |||||
| Zn2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 16.50 | |||||
| Cd2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 16.46 | |||||
| Hg2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 21.7 | |||||
| Pb2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 18.04 | |||||
| Al3+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 16.3 |
|
Fluoride \(\ce{F-}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Al3+ | 6.11 | 5.01 | 3.88 | 3.0 | 1.4 | 0.4 |
|
Hydroxide \(\ce{OH-}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Al3+ | 9.01 | [9.69] | [8.3] | 6.0 | ||
| Co2+ | 4.3 | 4.1 | 1.3 | 0.5 | ||
| Fe2+ | 4.5 | ]2.9] | 2,6 | –0.4 | ||
| Fe3+ | 11.81 | 10.5 | 12.1 | |||
| Ni2+ | 4.1 | 3.9 | 3. | |||
| Pb2+ | 6.3 | 4.6 | 3.0 | |||
| Zn2+ | 5.0 | [6.1] | 2.5 | [1.2] |
|
Iodide \(\ce{I-}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Ag+ | 6.58 | [5.12] | [1.4] | |||
| Cd2+ (T = 18 °C) | 2.28 | 1.64 | 1.08 | 1.0 | ||
| Pb2+ | 1.92 | 1.28 | 0.7 | 0.6 |
|
Nitriloacetate
|
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Mg2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 5.41 | |||||
| Ca2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 6.41 | |||||
| Ba2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 4.82 | |||||
| Mn2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 7.44 | |||||
| Fe2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 8.33 | |||||
| Co2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 10.38 | |||||
| Ni2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 11.53 | |||||
| Cu2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 12.96 | |||||
| Fe3+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 15.9 | |||||
| Zn2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 10.67 | |||||
| Cd2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 9.83 | |||||
| Pb2+ (T = 20 °C, \(\mu = 0.1 \text{ M}\)) | 11.39 |
|
Oxalate \(\ce{C2O4^{2-}}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Ca2+ (\(\mu = 1 \text{ M}\)) | 1.66 | 1.03 | ||||
| Fe2+ (\(\mu = 1 \text{ M}\)) | 3.05 | 2.10 | ||||
| Co2+ | 4.72 | 2.28 | ||||
| Ni2+ | 5.16 | |||||
| Cu2+ | 6.23 | 4.04 | ||||
| Fe3+ (\(\mu = 0.5 \text{ M}\)) | 7.53 | 6.11 | 4.83 | |||
| Zn2+ | 4.87 | 2.78 |
|
1,10-phenanthroline
|
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Fe2+ | 20.7 (\(\beta_3\)) | |||||
| Mn2+ (\(\mu = 0.1 \text{ M}\)) | 4.0 | 3.3 | 3.0 | |||
| Cu2+ (\(\mu = 0.1 \text{ M}\)) | 7.08 | 6.64 | 6.08 | |||
| Ni2+ | 8.6 | 8.1 | 7.6 | |||
| Fe3+ | 13.8 (\(\beta_3\)) | |||||
| Ag+ (\(\mu = 0.1 \text{ M}\)) | 5.02 | 7.04 | ||||
| Zn2+ | 6.2 | [5.9] | [5.2] |
|
Thiosulfate \(\ce{S2O3^{2-}}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Ag+ (T = 20 °C) | 8.82 | 4.85 | 0.53 |
|
Thiocyanate \(\ce{SCN-}\) |
log \(K_1\) | log \(K_2\) | log \(K_3\) | log \(K_4\) | log \(K_5\) | log \(K_6\) |
|---|---|---|---|---|---|---|
| Mn2+ | 1.23 | |||||
| Fe2+ | 1.31 | |||||
| Co2+ | 1.71 | |||||
| Ni2+ | 1.76 | |||||
| Cu2+ | 2.33 | |||||
| Fe3+ | 3.02 | |||||
| Ag+ | 4.8 | 3.43 | 1.27 | 0.2 | ||
| Zn2+ | 1.33 | 0.58 | 0.09 | –0.4 | ||
| Cd2+ | 1.89 | 0.89 | 0.02 | –0.5 | ||
| Hg2+ | 17.26 (\(\beta_2\)) | 2.71 | 1.83 |