28: Eyring
- Page ID
- 148309
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Eyring Plots: Transition State Parameters Help Determine Mechanism
How do we get information about the transition state?
The Eyring equation in chemical kinetics relates the reaction rate to temperature.
- As the temperature is raised, the reaction rate ______________.
- Explain this trend using collision theory.
- Draw a reaction profile for a simple reaction.
- Label
- Transition State
- ΔG‡ (akaEa)
In practice, activation energies are not often cited in the current literature. Instead, the Eyring equation is used to determine entropy of activation (ΔS‡ )andenthalpy of activation (ΔH‡ ):
$$
\ln (\mathrm{k} / \mathrm{T})=-\Delta \mathrm{H}^{\ddagger} / \mathrm{RT}+\Delta \mathrm{S}^{\ddagger} / \mathrm{R}+\ln \left(\mathrm{k}_{\text { Boltzmann }} / \mathrm{h}\right)
\]
where
k = rate constant
T = temperature in Kelvin
R = gas constant, 8.314 J/mol K
kBoltzman is Boltzmann’s constant, 1.38 x 10-23 J/K h is Planck’s constant, 6.6 x 10-34 J s
- What data should be plotted to make an Eyring plot?
- What is equal to the slope? Intercept?
Eyring Plots: Use in Mechanism Determination
- Draw an SN1 mechanism.
- Make a prediction about the size/sign of ΔH‡ :
Large & Positive | Small & Positive | Small & Negative | Large & Negative
- Make a prediction about the size/sign of ΔS‡:
Large & Positive | Small & Positive | Small & Negative | Large & Negative
- Draw an SN2 mechanism.
- Make a prediction about the size/sign of ΔH‡ :
Large & Positive | Small & Positive | Small & Negative | Large & Negative
- Make a prediction about the size/sign of ΔS‡:
Large & Positive | Small & Positive | Small & Negative | Large & Negative
Summary of Eyring Plots:
The Eyring equation is used to determine entropy of activation (ΔS‡) and enthalpy of activation (ΔH‡):
$$
\ln (\mathrm{k} / \mathrm{T})=-\Delta \mathrm{H}^{ \ddagger} / \mathrm{R} \mathrm{T}+\Delta \mathrm{S}^{\ddagger} / \mathrm{R}+\ln \left(\mathrm{k}_{\text { Boltzmann }} / \mathrm{h}\right)
\]
- What are the x- and y-axes in an Eyring plot?
- What is equal to the slope? Intercept?
- In the rate determining step of unimolecular reaction, the entropy (increases OR decreases) and ΔS‡ is ( positive OR negative ).
- In the rate-determining step of a bimolecular reaction, two molecules become one and ΔS‡ is_______________.
[In reality, care must be taken in interpreting ΔS‡ values near 0 (between +/- 40 J/ mol-K) because solvent reorganization may contribute to ΔS‡ ,especially for polar solvents and charged intermediates. ΔH‡ can be less useful. It is always positive, since in any mechanism there must be bond breaking or at least bond weakening as the transition state is reached.
Eyring Plots: Mechanism Determination in Ligand Substitution Reactions
In the case of ligand substitution in transition metal complexes, there are really four possible mechanisms. The two we have discussed this far represent the extremes—a D (dissociative) mechanism or an A (associative) mechanism.
- Draw an example of a dissociative ligand substitution.
- Predict the value of the ΔS‡ and the ΔH‡
- Draw an example of an associative ligand substitution.
- Predict the value of the ΔS‡ and the ΔH‡
There is a third possible mechanism. An “interchange” (I) mechanism is a more concerted ligand exchange.
- Suggest a mechanism for IA Ligand Substitution.
- What would you expect the sign of DS to be in a interchange mechanism? Explain.
Summary of Eyring Plots:
The Eyring equation is used to determine entropy of activation (ΔS‡ ) and enthalpy of activation (ΔH‡)
$$
\ln (\mathrm{k} / \mathrm{T})=-\Delta \mathrm{H}^{ \ddagger} / \mathrm{R} \mathrm{T}+\Delta \mathrm{S}^{\ddagger} / \mathrm{R}+\ln \left(\mathrm{k}_{\text { Bolzmann }} / \mathrm{h}\right)
\]
- What are the x- and y-axes in an Eyring plot?
- What is equal to the slope? Intercept?
- In the rate determining step of unimolecular reaction, the entropy ( increases / decreases ) and ΔS‡ is ( positive / negative ).
- In the rate-determining step of a bimolecular reaction, two molecules become one and ΔS‡ is ( positive / negative ).
[In reality, care must be taken in interpreting ΔS‡ values near 0 (between +/- 40 J/ mol-K) because solvent reorganization may contribute to ΔS‡, especially for polar solvents and charged intermediates. ΔH‡ can be less useful. It is always positive, since in any mechanism there must be bond breaking or at least bond weakening as the transition state is reached.
Application problems
- The rate of exchange free cyanide for bound cyanide in [Pt(CN)4]2- was monitored via 13C NMR and found to have activation parameters of ΔH‡ = 25.1 kJ/mol and ΔS‡ = -142 J/mol-K. What is the geometry at Pt? What do the activation parameters suggest about the mechanism? (Inorg Chem. 2002, 42, 1717.)
- Propose a mechanism for the following reactions.
[Ru(EDTA)(H2O)]- + L -> [Ru(EDTA)L]x + H2O
Ligand k (relative) ΔH‡ (kJ/mol) ΔS‡ (J/mol-K) Pyrazine 20,000 5.7 -20 Isonicotinamide 8,300 6.6 -19
Pyridine 6,300 - - Imidazole 1,860 - - SCN- 270 8.9 -18 CH3CN 30 8.3 -24 - The following reaction, with different benzene substituents and in different solvents, was studied experimentally and via chemical computation. What would you predict to be the mechanism of the reaction? Are the activation parameters consistent with your proposal? (Ruff et al, J. Org. Chem. 2006, 713409-16.)

- Therateconstantandactivationparametersforthereplacementofpyridineby various solvent molecules in [Pd(Me5dien)py]2+ were reported. (Kotowski, et al.Inorg. Chem. 1988, 27, 4472-4474.) (Dien is a tridentate version of ethylenediamine.)
Solvent 105 x k ΔH‡ ΔS‡ EtOH 7.4 86 -38 MeOH 4.1 87 -39 H2O 1.8 69 -105 MeSO 53 69 -78 DMF 90 63 -91 MeCN 1310 60 -77 - What does the variation in rate constants with solvent say about the mechanism?
- What about the activation parameters?
- Can you come up with an explanation for why certain of the solvents make the substitution reaction faster? (What do the fast ones have in common? The slower ones?)
- The following kinetic data was obtained for the reaction of trans-[Co(NH3)4Cl2]Cl + H2O -> cis-[Co(NH3)4(H2O)(Cl)]Cl2.
- Based on electron count and sterics and geometry, what type of ligand substitution would you predict for this reaction?
- Use Excel to make an Eyring plot to calculate the activation parameters.
T(C) T (K) 1/T (K-1)
k ln (k/T) 19 292 0.003425 6.60E-04 -13.000 20 293 0.003413 7.30E-04 -12.9026 21 294 0.003401 8.20E-04 -12.7898 22 295 0.00339 9.60E-04 -12.6356 23 296 0.003378 1.16E-03 -12.4497 24 297 0.003367 1.34E-03 -12.3088 25 298 0.003356 1.51E-03 -12.1927 26 299 0.003344 1.71E-03 -12.0717 - What type of mechanism do the activation parameters indicate for this reaction?
- In a classic mechanistic study (Organometallics, 1989, 8, 2144), Basoloandco- workers investigated the following reaction:
M(CO)5 + (CH3)3NO + PPh3 -> M(CO)4(PPh3) + (CH3)3N + CO2
(M=Fe, Ru, Os)
They reported the following findings:
- the reaction was first order in both complex and (CH3)3NO and 0th other in PPh3
- for M=Fe, the activation parameters were determined: ΔH‡ = 14.5 kcal/mol and ΔS‡ = -16.5 cal/mol K
- Write a step-by-step mechanism that is consistent with their findings.
- Which one is the slow step?
- Draw a Lewis dot structure for (CH3)3NO and draw an “arrow pushing diagram” to show how/why it likely is interacting with the metal complex in the way that it does. (That is, what is the structure of the activated complex involving these two species, and how will the electrons move to form the product of that step?)
- The authors note that for these three and other similar complexes, the rate of the reaction increases with the increasing CO stretching frequencies in the starting material. Explain why this might be. (Your diagram above may help.)
- They go on to note in the paper that the relative rate of reaction is Os>Ru>Fe. Suggest a reason for this pattern.
- The following reaction was studied and the activation parameters obtained. Propose a mechanism for the debromination reaction. Butcher, et al; J. Org.
Chem., 2009, 64, 5677-5681. ΔH‡ =22 kcal/mol and ΔS‡ =-26 cal/mol-K

- What is the role of the Te compound in this reaction? (Since Te is in the same family as O, the analogous oxygen compound would be an ether, which would be unlikely to cause this reaction. Why does Te? Hint—draw a Lewis dot structure for the Te-containing product.)
Eyring Plots in Polymer Chemistry
Example 1: Mechanism for Deactivation of Ni(II) Polymer Catalysts
Polyethylene is the most common plastic. The annual production is approximately 80 million metric tons. Its primary use is in packaging (plastic bag, plastic films, containers including bottles, etc.).
Olefin polymerization by cationic complexes of d8 metals (late transition metals) has been studied intensely in the past decade. These catalysts are much more tolerant toward polar reagents in general than are early transition metal counterparts.
- What metal was used in the Ziegler-Natta process?
- Why would it be industrially important to use a catalyst that can be used in polar reagents/solvents?
In addition to their tolerance for polar environments, neutral Ni(II) complexes have unique synthetic utility including high polymerization rates and controllable degrees of polymer branching (leading to crystallinity). Thus, a quantitative study of the kinetics and intermediates that lead to deactivation of the catalyst can be important for improving polymerization catalysts.
Berkefeld and Mecking, J. Am. Chem. Soc., 2009, 131, 1565-1574.
- Propose a catalytic cycle for this polymerization. Show at least two turns around the cycle. Note: L2 is an anionic (-1) bidentate ligand.

Mechanism for deactivation of Ni(II) Polymer Catalysts (cont)
The proposed methods for deactivation of the Ni(II) catalyst is shown below:

- What is the rds of this mechanism?
- Predict the molecularity of this proposed mechanism.
The Eyring data yields a ΔH = 57 kJ mol-1 and an ΔS = -129 J mol-1K-1, corresponding to a free energy of activation of ΔG = 95 kJ mol-1.
- What does this data indicate about the mechanism?
- When this catalyst is stored in CH2Cl2 it slowly decomposes. When the catalyst is stored in DMSO, it is stable. Suggest a reason for this difference.
Example 2: Reactivity of Methacrylates in Polymerization
Catalytic polymerization of ethylene is one of the most well-studied chemical reactions. In terms of applications, it is employed for the production of more than 70 million tons of polyolefins annually. An insertion (co)polymerization of electron- deficient polar-substituted vinyl monomers like acrylates remains a challenge, however. It was not until the mid-1990s that Pd(II) complexes were reported to catalyze the insertion copolymerization of ethylene with acrylates.
Rünzi, Guironnet, Göttker-Schnetmann and Mecking, J. Am. Chem. Soc., 2010, 132, 16623-16630.
- Draw two or three cycles of polymerization of ethylene (CH2CH2) with this catalyst:

- Draw the co-polymer formed when this catalyst adds monomers of ethene and methyl acrylate (MA).
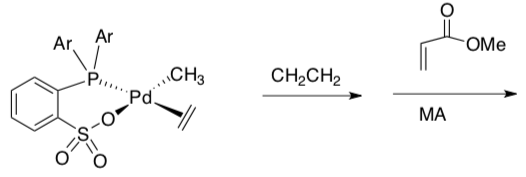
- The catalyst in the presence of ethylene and methylmethacrylate (MMA) produces only polyethylene. Suggest a reason why the MMA is not incorporated into the growing polymer.

The authors completed Eyring plots for the disappearance of the catalyst when reacted with MMA (left) and MA (right).

- Calculate the ΔG‡ for each reaction. Which has a higher barrier?
The authors took advantage of this differential reactivity of MA vs MMA by using a bi-functional monomer.
- Draw the co-polymer formed when this catalyst adds monomers of ethene and the bifunctional acrylate monomer shown.

- Is there likely to be cross-linking? Why or why not?
In post-polymerization modification, a polymer can be turned into a diverse range of other polymers simply by modifying side chains.
- Suggest a method for post-polymerization modification.


