27: Inorganic polymers
- Page ID
- 148288
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Introduction to Main Group Chemistry
Passport – Main Group Chemistry
“Main group” = non-metal non-carbon chemistry.
One of the factors that controls reactivity is thermodynamics. Let’s compare some bond strengths involving C and Si.
| Bond type | Energy (kJ/mol) | Bond type | Energy (kJ/mol) |
| C—C | 347 | Si—Si | 226 |
| C=C | 611 | Si=Si | 318 |
| C—O | 360 | Si—O | 464 |
| C=O | 732 | Si=O | N/A* |
| C—F | 490 | Si—F | 598 |
| C—Cl | 330 | Si—Cl | 381 |
*Si=O bonds are very uncommon—the first compound containing one was not prepared until very recently.
Carbon is the element most known for forming bonds to itself. (This is calledcatenation.) Silicon is in the same family as C.
- Based on the data above, do you think it is similar in this regard? Explain.
- Do you think there are a lot of compounds with Si=Si bonds? Explain.
- As you know, C commonly forms both single and double bonds to O. Give a thermodynamic argument as to why this is so.
In contrast to what we have seen thus far, the Si-O bond is stronger than the C-O bond. The explanation for this is that in addition to the “normal” Si-O sigma bond, there is a second component to the bond: a lone pair on O (assume in a p orbital) is donated into
an empty d orbital on Si. This is called a dπ-pπ bond.
- Draw the orbitals overlapping to create this interaction.
- Why doesn’t this happen in C chemistry?
The Si-F bond is generally considered to be one of, if not the strongest, “single” bonds known.
- Explain why.
The Si-Cl bond is considerably weaker than Si-F.
- Suggest a reason why this may be.
The second general factor to consider in analyzing reactivity is kinetics. (There has to be a relatively low energy route to products.) Again, let’s compare Si to C. Consider CH2Cl2, which is fairly inert. SiH2Cl2, on the other hand, is very reactive. Since the bonds to Cl and H are of comparable strength in both C and Si chemistry, it is unlikely that the answer lies in thermodynamics. However, it is likely that the Si is more easily attacked.
- Name a property that changes when we go from C to Si in the periodic table that might explain why Si is more easily attacked.
- Propose a mechanism for attack based on earlier data about Si chemistry.
- Now explain why R2Si=SiR2 and R2Si=O compounds are kinetically less stable than their carbon counterparts.
As a rough rule of thumb, the reactivity of Si-Cl bonds (and other main group-Cl bonds, such as B-Cl, P-Cl, etc) is very similar to the reactivity of the 0=C-Cl bond.
Inorganic Polymers
Inorganic polymers are polymers with a skeletal structure that does not include carbon atoms. One of the best known examples is polydimethylsiloxane.
Its applications range from contact lenses and medical devices to shampoos (it makes hair shiny and slippery), food (antifoaming agents), caulking, lubricating oils, and heat- resistant tiles.
It has a repeat unit shown below:
—[O-Si(CH3)2]n—
- Propose the structure of the starting monomer. Think about leaving groups!!
Industrial synthesis can begin from dimethyldichlorosilane and water by the following net reaction:
n Si(CH3)2Cl2 + n H2O
- Draw a mechanism for this reaction. Remember the hexavalent intermediate required for substitution on a silicon center.
- Draw a 6-mer of this polymer.
Silicon Polymers: Silly Putty Case Study
The starting material in the preparation of silly putty is (CH3)2SiCl2. In the first step of the reaction, this compound is dissolved in ether, and water is carefully added.
- Predict the product of this reaction.
Instructions for the procedure indicate that the reaction should be performed in a fume hood. This is because of the other product of the reaction.
- What is the by-product?
One family of products of this reaction are cyclic structures with the formula [(CH3)2SiO]x (x is most commonly 3-5).
- Draw the product when x=3.
There are few or no molecules formed where x=2.
- Draw that species, and explain why its formation is unlikely.
In addition to the cyclic structures, there are also linear silicone species that terminate in an –OH on each end.
- Why do you suppose these do not tend to cyclize to create a ring with 20, 50 or 100 silicone units in it?
The ether solvent is then removed to leave this mixture of products, which is called a silicone oil due to its consistency. In the final stage of the reaction, this oil is mixed with approximately 5% by weight boric acid (B(OH)3) and heated to 200°C. This temperature causes the smaller rings to open and link with each other and the linear species to make longer chains.
- Write a mechanism to show how reaction with boric acid might cause a cyclic silicone structure not only to open but to place a –B(OH)2 group at the end of the chain. (Hint—there are at least three different reasonable mechanisms one might propose.)
Large molecules tend to be more viscous than smaller ones.
- Explain this using your understanding of polymer architecture.
However, if the chains get chemically attached to each other the viscosity will increase even more. This formation of bonds between chains is called cross-linking and results in this case in the formation of the gum-like material known as silly putty.
- Explain, using structures, how adding boric acid will promote cross-linking.
If a ball of silly putty is left on a table, it will “flow”, over the course of minutes or hours, to create a flat film.
- Explain what is happening on the molecular level to allow this.
Suppose that 10% by weight boric acid were added to the silicone oil instead of the typical 5%.
- How would the properties of the final product change?
You have also encountered silicone polymers as bathtub or weatherproofing caulk or aquarium sealants.
- Given the physical characteristics of these products, what would you conclude about the extent of crosslinking in them?
- Suggest two ways to promote cross-linking.
Silly putty/silicone fun facts (great to break the ice at parties!)
- When newspapers employed petroleum-based inks, silly putty could be used to pick up an image such as a comic character and stretch it. With current soy-based inks this no longer works.
- Since 1950, more that 300 million silly putty “eggs” have been sold.
- Apollo astronauts used silly putty to hold tools in zero gravity.
- Despite strong sales, the original toy store owner that sold silly putty decided to drop the product in 1950. An advertising consultant saw the opportunity, and although $14,000 in debt, borrowed $147 to obtain a batch of silly putty. He had Yale students separate it into 1 ounce balls and place them in egg-shaped containers. The rest is history. In 1976 when he died his estate was valued at $140 million. (What’s the lesson here?)
- Because of their low toxicity, resistance to high temperatures, low reactivity, etc, silicone polymers have made their way into a number of consumer and scientific products including insulating mitts (the “ov glove”), stopcock grease, etc. Silicone in hair products makes it soft and removes tangles and frizz. Silicones are antifoaming agents, both industrially and to reduce bloating due to excess gas in the stomach and intestine. Silicones serve as anticaking agents (prevents solids such as salt from forming lumps) and are present in Chicken McNuggets. Look for silicone, dimethicone or simethicone or cyclomethicone on the product label.
- Many personal care products contain the cyclic tetramer or pentamer. Because of concerns that the tetramer tends to bioaccumulate and contaminate wastewater, the pentamer is now much more commonly used.
Polyphosphazene polymers
The polyphosphazines are a large and diverse family of polymers with many practical applications. This activity will give you an understanding of these molecules.
- Draw the Lewis dot structures for these two molecules and determine the hybridization of the central atoms.
PCl5 | NH3
- Predict the product when 1 PCl5 reacts with 1 NH3 to form a white solid.
The white solid is now known to have the formula and structure shown below. It is called hexchlorotriphosphazine.
- Add any lone pairs necessary to complete the structure.
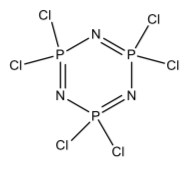
- Does Huckel’s Rule predict that it is aromatic?
- What is the hybridization of the P and N?. What orbitals, then, form the pi bond between P and N?
Below are the six atoms in the ring written in a line. (N1 must also connect to P3.)
- Now draw in the atomic orbitals on each , including appropriate shading, that form the lowest energy pi MO. What does this suggest about the aromaticity of the molecule?
N1 | P1 | N2 | P2 | N3 | P3
- is there any delocalization? In these molecules it is sometimes said that there are “islands” of delocalization. Explain what this means.
Shortly after its original discovery, it was noted that heating the material produced a white substance that was dubbed “inorganic rubber” due to its consistency. However, since the material was insoluble in all laboratory solvents it was not studies extensively until a major breakthrough in the 1960’s. At that point a set of reaction conditions was developed that allowed the cyclic structure to reproducibly open to form a linear polymer with a consistent molecular weight. These reaction conditions are given below:
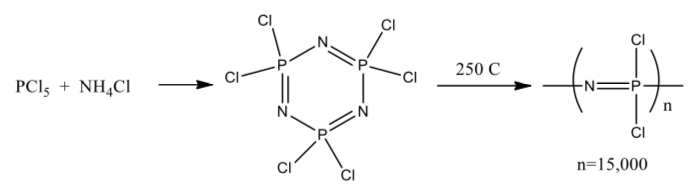
The linear polymer is soluble in solvents such as benzene, toluene and THF.
- Draw two such rings. Suggest a possible mechanism for ring opening to form the polymer. (Hint—It was also discovered that ring opening was accelerated by trace amounts of AlCl3 or BCl3).
This linear polymer is not very useful because of the hydrolytic instability of the P-Cl bonds. However, this reactivity allows the chlorines to be replaced with a wide variety of nucleophiles! The side groups of the polymer control solubility, hydrolytic stability, thermal stability and a wide variety of optical, electrical and biological properties. For example, one of the first polymers prepared was the following:

This polymer is microcrystalline, forms films and fibers easily, is stable to water and hydrophobic. It is presently used in artificial heart valves and blood vessels.
- What is the other product of this reaction? Explain why its formation (in toluene) helps drive this reaction forward.
The polymer backbone itself imparts flexibility, fire resistance and stability.
- Suggest a reason why it might be more fire resistant than an organic polymer.
There are two possibly configurations of the backbone, the so-called trans-trans-planar and the cis- trans-planar. These structures are shown below. Virtually all polyphospahazines have been found to behave the same structure.
- Which of the two possible configurations do you think it is? Why?
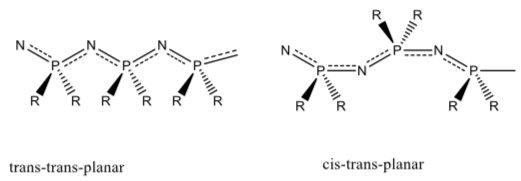
The flexibility attributed to the polymer backbone might seem unusual.
- Why might you predict that the polymer backbone should be rigid?
- What is the hybridization of the P in the polymer? What type of orbital on P is forming the double bond to N? How many unused orbitals of this type on P are there? Suggest a reason why this might produce a lower-than-expected barrier to rotation in this system.
Theoretical studies have suggested that the barrier to rotation is only 0.5 kcal/mol.
- How does this barrier compare to what we would expect in a “true” double bond? Suggest a reason why the barrier in the polyphosphazines might be smaller.
A close connection exits between the backbone flexibility and the rigidity of the polymer material. In polymer chemistry, the glass transition temperature (Tg) is a measure of polymer flexibility. Below Tg, there is insufficient thermal energy for the polymer backbone to rotate and the polymer acts like a glass. Above Tg, the molecule can flex and rotate to relieve strain. The polyphosphazines have some of the lowest Tg’s known. For example, [NP(Cl)2]n has a Tg of -66 C and [NP(OC3H7)2]n is -100 C.
- Draw a structure of polypropylene [C2H3(CH3)]n, , which has a Tg of -10 C, and explain why the Tg for many of the polyphosphazines is significantly lower.
Application Problems
- Suppose that you wish to synthesize the silly putty analogue that has ethyl side groups instead of methyl. What is the starting material for the reaction?
- When discussing polyphosphazine substitutions you learned the reagents to use to form either P-O or P-N sidechain bonds. We have previously drawn the analogy between organic acid chlorides and main group E-Cl bonds. If this analogy is strictly accurate, what reagent would you use to replace the P-Cl bond with a P-C bond? Now name two other reagents that might be expected to do the same thing (and in fact are more commonly used.)
- Now give two syntheses for the starting material needed for the diethyl analogue of silly putty. In the first case, start with SiCl4. In the second, suggest a reaction similar to that needed to prepare a Grignard reagent.
- Heterocatenation is the formation of chains in which every other atom is the same. Analyze the statement, “While carbon is the king of catenation, other main group elements make an equal variety of molecules through heterocatenation.”
- Propose a synthesis for the bioerodable polymer [NP(NHCH2COOC2H5)2]n from any compound that has no P-N bonds. Then suggest a mechanism for the hydrolysis of the ester group and the polymer chain itself.
- Si-Cl bonds are more easily hydrolyzed that C-Cl bonds even though the former are stronger. Explain how this can be.
- In the synthesis of silly putty a possible impurity in the (CH3)2SiCl2 is (CH3)3SiCl. If the starting material in the silly putty synthesis did contain 5% of the monochloride how would the reaction product differ, both chemically and in physical properties? How would the product differ if the starting material contained 5% CH3SiCl3 instead?
- Silicone oils are used as stopcock grease in the chemistry lab, or in any application where two ground glass joints come into contact. Glassware with stopcock grease can be effectively cleaned by placing it in the “base bath”—a cleaning solution containing a high concentration of NaOH in ethanol and water. This mixture dissolves stopcock grease. Explain how. It doesn’t dissolve hydrocarbon grease. Why not?
- It was noted that the Si-O bond is extremely strong, due to the partial dπ-pπ bond.
However, the Si-O bond is also said to be extremely flexible, as evidenced by the ability of silly putty to “flow”. Explain how the bond can have partial double bond character and still be flexible. (Hint—think about the polyphosphazines.)


