26: Cationic Polymerizations
- Page ID
- 148287
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Cationic Polymerizations
Cationic Polymer Mechanisms
Polyisobutylene is a synthetic rubber, or elastomer. It is the only rubber that is gas impermeable. Because polyisobutylene will hold air, it is used to make things like the inner liner of tires and basketballs.
- Draw a curved arrow mechanism for the acid-catalyzed polymerization of 2-methylpropene (isobutylene).

- Draw a curved arrow mechanism for the polymerization of styrene.
- Show at least two of the polymer chain lengthening steps.
- Show at least two of the polymer chain lengthening steps.
Polyethylene is an important plastic used in the preparation of water bottles, toys, etc.
- Explain why the acid-catalyzed polymerization method does not work for the synthesis of polyethylene.

Because polyethylene and polypropylene cannot be made using acid-catalysis, a metal catalyst is used to make the commercially popular plastics such as polyethylene and polypropylene. This procedure earned Karl Ziegler and Giulio Natta the Nobel Prize in 1963.
- Show a curved arrow mechanism for the chain-lengthening reactions shown below. Fill in missing intermediates or names of missing mechanistic steps.

Tacticity
Tacticity refers to the stereochemical arrangements of groups along a polymer backbone.
- What are the stereochemical relationships among the following oligomers?

- What would you predict about differences in physical properties among these polymers?
Define the following terms:
- Isotactic
- Syndiotactic
- Atactic
Control of tacticity is a major objective in polymer chemistry. Like MW and PDI, tacticity has a major impact on polymer properties. For example, the Tg of the polypropylenes (above) have very different values:
Atactic: -20oC | Syndiotactic: -8oC | Isotactic: -1oC
- Propose a reason for these differences in mp.
Atactic Syntheses
- Cationic Polymerizations
- Predict the tacticity of the polypropylene resulting from the following cationic polymerization process.

- Predict the tacticity of the polypropylene resulting from the following cationic polymerization process.
- Titanocenes such as Cp2TiCl2 with AlMe3 result in a cationic system capable of polymerizing alkenes. (Jordan, J. Am. Chem. Soc. 1986, 108, 7410-7411).
- Draw the mechanism for this pre-catalyst step.
- Draw the side product.

Polymerization is thought to involve a series of binding and 1,2-insertion steps.
- Draw the mechanism for this polymerization.

- Why do you suppose this mechanism results in an atactic polymer?
Isotactic Polymerizations
- Ziegler-Natta Heterogeneous Catalysis
Among other advantages, Ziegler-Natta polymerization allows for control of stereochemistry in polypropylene. This mechanism appears to follow this pathway.
- Identify the chirality of the methyl after the first addition.
- Fill in missing intermediates to complete the addition of a second monomer.

- Why does the propene approach the titanium catalyst with the methyl pointing toward you?
- Why does this mechanism/catalyst result in an isotactic polymer?
Isotactic Polymerizations
- Symmetric homogeneous zirconocene catalysts
This chiral, wedge-like compound shown below catalyzes Ziegler-Natta polymerization of propene to provide even better isotactic stereocontrol.

Below, the catalyst has begun propene polymerization. This time, you can see the orientation when looking at the catalyst from in front of the wedge.
- Fill in the boxes for the continuing isotactic polymerization via 1,2-insertion.

- Fill in the boxes for the continuing isotactic polymerization via 1,2-insertion.
Syndiotactic Polymerizations
- Asymmetric homogeneous zirconocene catalysts
The following catalyst is shown oriented so we are looking from in front of the wedge.
- Show how a propene would bind to the empty site of the metal catalyst below.
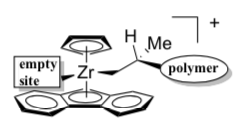
- Fill in the blanks on the following synthesis.

- Why does this catalyst structure lead to syndiotactic polymerization? Which face does the growing chain add to each time?
- Show how a propene would bind to the empty site of the metal catalyst below.
Living Cationic Polymerization
Living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed.
- For a typical cationic polymerization, the termination is usually an elimination step. Show a termination step for this growing chain.

- Fill in the blanks on the following synthesis of a living cationic polymer.

- Show a mechanism for the regeneration of the living chain from the dormant chain.
- What structural factors allow this reaction to be reversible?
This equilibrium can be controlled by solvent or salt concentration.
- Decreasing solvent polarity will favor:
Dormant | Living
- Increasing chloride ion (inert salt addition) will favor:
Dormant | Living
Living Polymerization Results
- In living polymerization, the growing chain spends much more time in the dormant phase
- there is a (higher / lower) likelihood of random termination steps.
- polymer chains grow at a ( more / less) constant rate than seen in traditional chain polymerization and their lengths remain very similar.
- Living polymerization is of interest because it produces polymers with ( low OR high ) polydispersive indices.
- Explain how the polymerization process affects the polydispersive index.
- Match these graphs of rate vs time with the type of polymerization:

Testing to Determine Living Polymerization Chain Growth
When a chemist is trying to determine whether a polymerization is proceeding through living polymerization, there are two primary approaches to use.
- Graph MW vs [monomer]
- Which of these lines would indicate a living polymer?
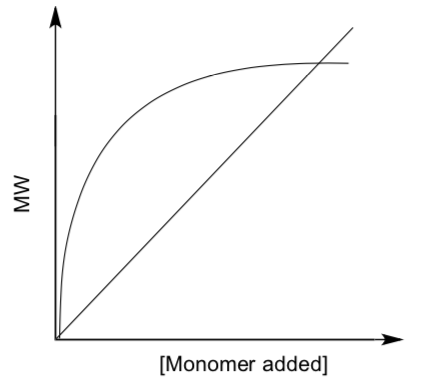
- Which of these lines would indicate a living polymer?
- Graph MW vs PDI
- Which of these lines would indicate a living polymer?
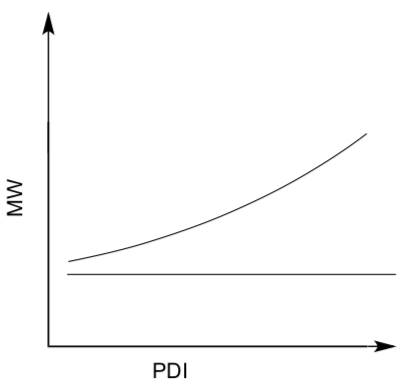
- Which of these lines would indicate a living polymer?
Living Polymerization (almost cationic)
Another approach for controlling chain growth using living polymers is to use insertion reactions (partial cation) rather than cationic reactions. This approach produces a polymer with a polydispersive index of 1.1.
- Draw the product for this reaction.

- How reactive is this resonance-stabilized cation?
Not reactive | A Little Bit | Medium Reactivity | V. Reactive
The reaction can be activated to polymerize by the addition of ZnI2.
- Draw a curved arrow mechanism for this polymerization.
- Complete the missing intermediates.

This approach produces a polymer with a polydispersive index of 1.1.
- What does this index tell you about the lengths of the resultant polymer?
Chain Growth and Length
Because the very controlled chain growth, you can determine the MW of the polymer simply by the ratio of the monomer to initiator.
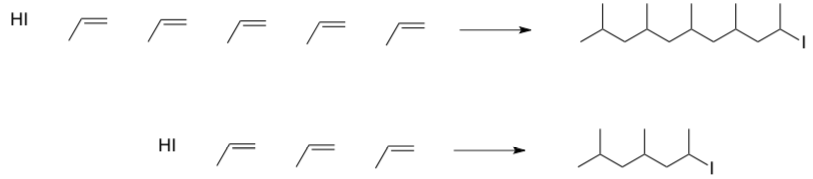
- Explain how this ratio determines the polymer length.
Kinetics of Living Polymerization Chain Growth
For the same reaction:
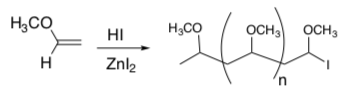
- What species is in the rds?
- What is the rate law for this reaction?
- Graph:
- Rate vs [Monomer]
- Rate vs [ZnI2]
- Rate vs [HI]
Summary of Cationic and Living Polymers
- Definitions:
- Step Growth:
- Chain Growth:
- Show an example of a cationic polymerization.
- Show the mechanism of the Ziegler-Natta Polymerization.
- Explain how living polymerization can control the rate of polymerization.
- Explain how living polymerization process affects the polydispersive index.
Summary of Polymer Structure and Properties
- Definitions:
- Tg:
- PDI:
- Isotactic
- Syndiotactic
- Atactic
- Degree of Polymerization


