22: E+ Additions
- Page ID
- 148176
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCElectrophilic Additions: E+ Additions with a Carbocation Intermediate
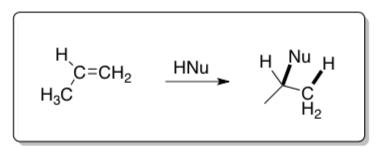
Electrophilic Addition Mechanism
In electrophilic addition, the p electrons of alkenes or alkynes act as the nucleophile. A cation is formed which subsequently reacts with an available nucleophile in solution.
- Are π or σ electrons held more tightly according to Coulomb’s Law? Explain.
- Draw the arrows for the mechanism of propylene undergoing electrophilic addition.
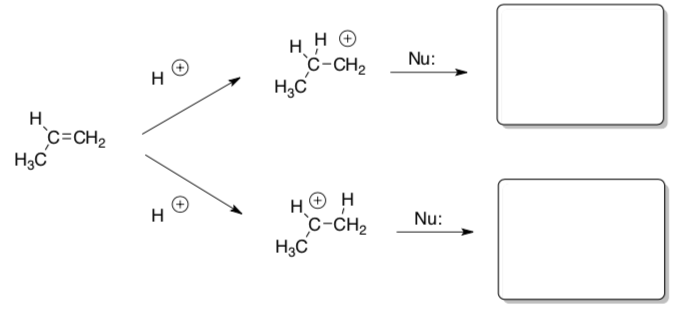
There are two possible cationic intermediates possible in the two additions above.
- Which of the cations produced is more stable? Why?
- Review: List at least 2 structural features that lead to stable carbocations.
- Circle the favored product in the reaction above.
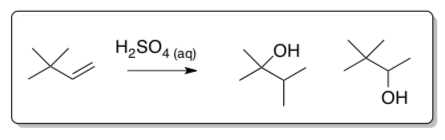
Relative Rates of Hydration:
- Draw the starting alkene and product for each of these hydration reactions in aqueous sulfuric acid.
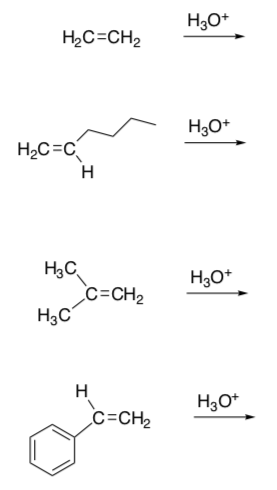
- Explain the difference in the reaction rates for these reactions.
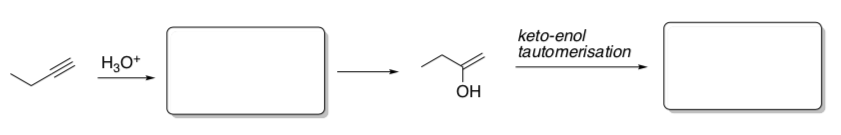
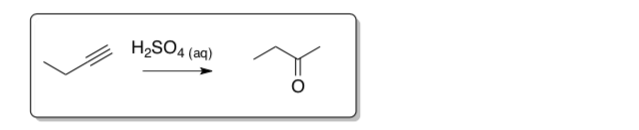
Alkene krel ethene 1 1-hexene 107 2-methylpropene 1012 phenylethene 109 - Alkynes can also be hydrated under aqueous acidic conditions. Draw the hydration mechanism of 1-butyne in H2SO4 and water.
Rearrangements of Carbocation Intermediates
Carbocations can undergo 1,2 hydride shifts or 1,2 methide shifts.
- Draw a mechanism for the following reaction including the cationic intermediate. Be sure to explain the presence of both products.
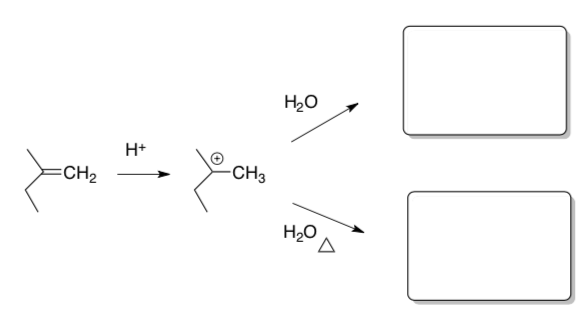
Competition between Addition and Elimination
Hydrations of alkenes proceed through a carbocation intermediate.
- What other reactions proceed through a carbocation intermediate?
Once the carbocation is formed, the reaction can proceed with addition of a nucleophile or undergo elimination (like E1).
- Show two possible products for this reaction under acidic conditions.
- What will favor the elimination?
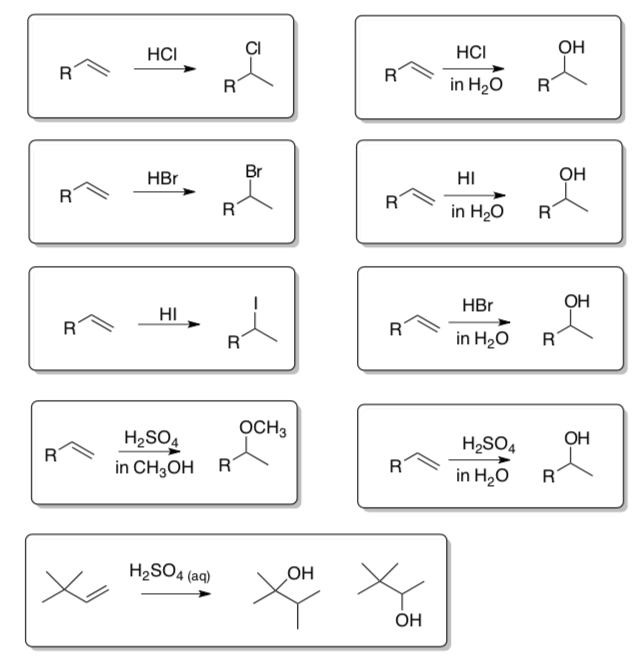
Acid Catalyzed Electrophilic Reactions
- Make Notecards of all new reactions!
Summary of Acid Catalyzed Additions
- Draw a generic mechanism of an acid catalyzed elelctrophilic addition reaction.
- What is the intermediate in all of these reactions?
- When reactions have cation intermediates, is there any stereocontrol?
- Draw an example of an electrophilic addition that undergoes a rearrangement.
- When will rearrangements occur?
Practice Problems:
- Provide a mechanism for the following formation of an ester:
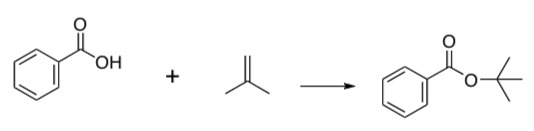
- Design a synthesis of MTBE using electrophilic addition to an alkene (HINT: alcohols are nucleophiles).

- Provide a mechanism using arrow formalisms for the following change:
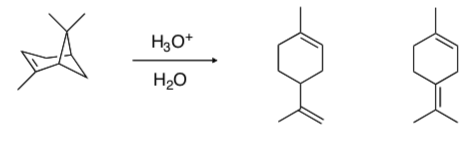
When the source of acid is carefully purified HBr, two bromides are also produced in the reaction above.
- Draw these products. Be careful, the two compounds are structural isomers, not stereoisomers.
- Conjugation
- Draw the product of 1,3-butadiene reacting with HBr.
- Draw any reasonable resonance structures for the cationic intermediate.
- Draw a Hückel MO diagram of allyl cation.
- Using this MO diagram, explain why the bromide ion can add in either the 1 position or the 3 position.
When this reaction is run at or below room temperature it normally leads to a mixture of products in which the 3-bromo-1-butene predominates over 1-bromo-2- butene. When the same reaction is carried out at higher temperatures, though, the product ratio often changes and the 1-bromo-2-butene predominates over the 3- bromo-1-butene. The product ratios at different temperatures are shown below.
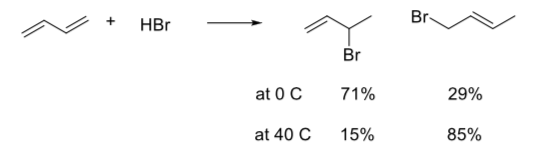
- Which is the kinetic product? Which is the thermodynamic product?
- Draw a general potential energy diagram for the two pathways of this exothermic reaction. (Don’t worry about exact energies). Label each pathway.
- Furthermore, when the product mixture formed at 0° C is reheated to 40° C, the ratio of products slowly changes from 71:29 to 15:85. Why?
Electrophilic Additions: Cyclic Bromonium/Mercuronium Intermediates
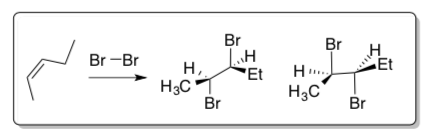
Anti-addition and stereochemical outcomes
Bromination of alkenes does not proceed through a carbocation intermediate.
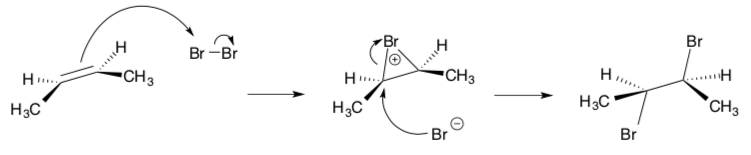
It is known that this reaction yields exclusively the enantiomeric pair shown below. This is known as anti-addition.
- Draw this mechanism. Explain why the syn product is not observed.
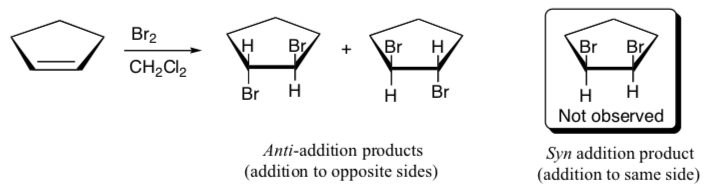
- What would be the product of the reaction of Br2 with the following alkenes? Show stereochemistry!
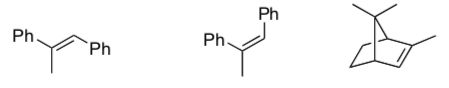
- Would you expect rearrangements to be possible with this mechanism? Explain.
Oxymercuration
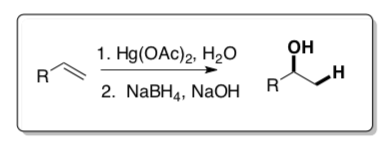
Oxymercuration-demercuration is a two-step process: 1) electrophilic addition of Hg+2 and a nucleophile and 2) demercuration.
Step One: Hg(OAc)2 can react in a similar fashion to Br2.
- Mercury is in ____________oxidation state thus it is a good ( electrophile/nucleophile), The acetate (CH3COO—) is a ( good / bad ) leaving group.
- Show a mechanism for an alkene reacting with Hg(OAc)2 and H2O. Be sure to show the cyclic mercurinium intermediate formed.
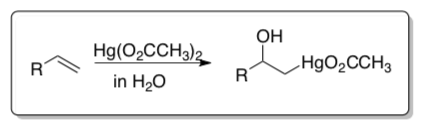
- Hg+2 is d__________.
Given this electron configuration, to which orbital will the alkene donate electrons.
- Is the product (mercury complex) labile or inert?
Step 2: The mercury can be removed from the molecule using NaBH4 in NaOH.
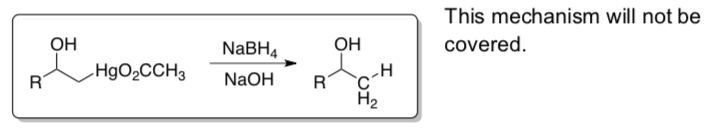
Combined:
- Fill in the boxes for the oxymercuration-demercuration of 1-hexene.
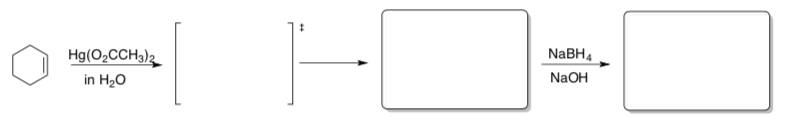
Practice with variations!
- Predict the product for these variations on bromination and mercuration. Include stereochemistry in your products.
Summary of Additons with Cyclic Intermediates
• Make notecards!!
You have two different mechanisms for Electrophilic Additions:
- Cationic Intermediate
- Cyclic Bromonium/Mercuronium Intermediate
- Draw the two different intermediates.
- Which type of intermediate allows for rearrangements?
Cationic | Cyclic Intermediate | Both | Neither
- Which type of intermediate leads to complete loss of stereocontrol?
Cationic | Cyclic Intermediate | Both | Neither
- With a carbocation intermediate, where does the -OH or -Br substituent add?
More Substituted C | Less Substituted C | Anywhere it feels like it
- With the cyclic intermediate, where does the -OH or -Br substituent add?
More Substituted C | Less Substituted C | Anywhere it feels like it
Electrophilic Additions
Concerted Additions (No Carbocation intermediate)
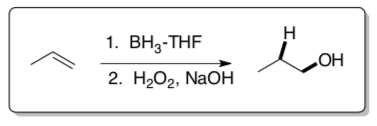
Hydroboration/Oxidation
These reactions were developed by H.C. Brown at Purdue University. He received a Nobel Prize for his work with boron compounds.
- For the two reactions shown below:
- Label each carbon in the transition states with ��+ and ��-.
- Identify sites of steric interaction in the transition states.
- Draw the product formed.
- Explain why there is a preference for the second transition state.
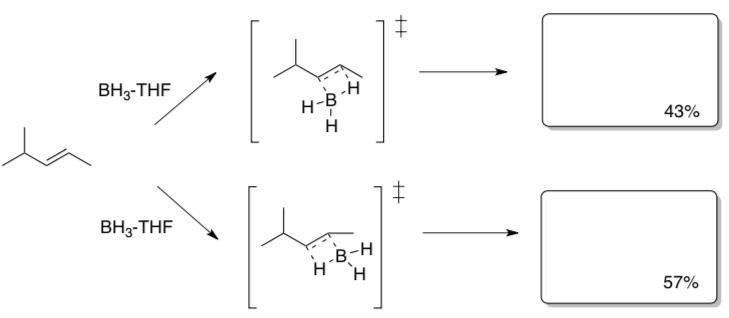
Conclusion: The boron substituent ends up on the (more or less) ____________substituted end of the alkene.
Boranes are typically oxidized to the corresponding alcohols. It is important to note that this oxidation proceeds with retention of stereochemistry as is shown below. You will not be expected to know this mechanism, but you should know the conditions and results.
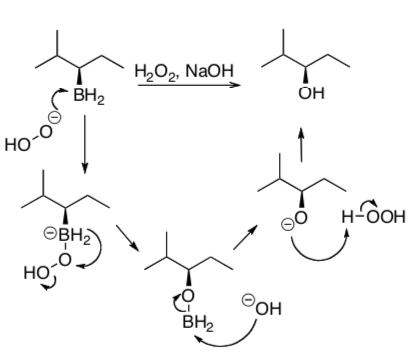
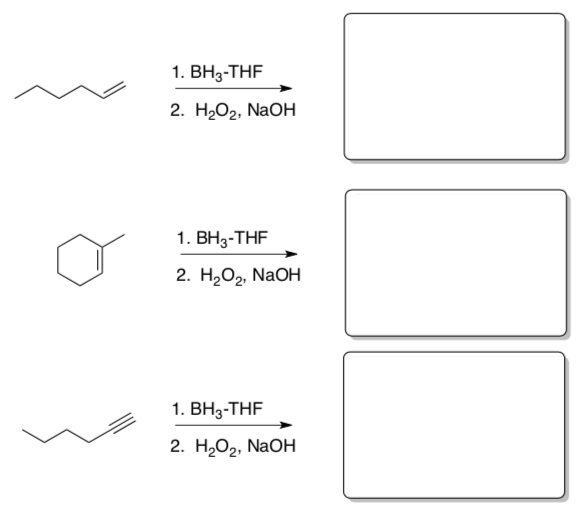
Practice the two-step!
Regiochemical Control of Hydroboration
When this reaction carried out with different borane reagents, the following product ratios are obtained.
- Show drawings of the transition states.
- In the transition states, circle significant sites of steric hindrance.
- Explain the difference in percentages of products with different boron reagents.
Transition State
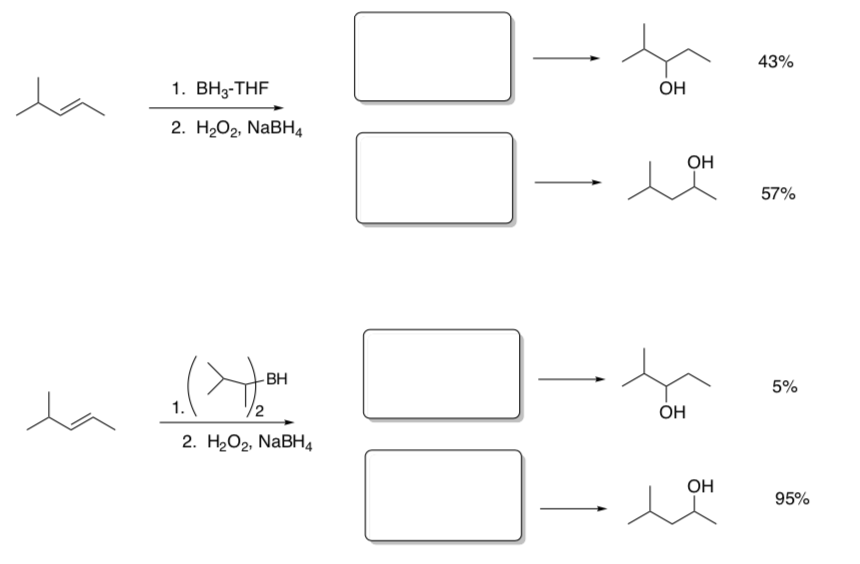
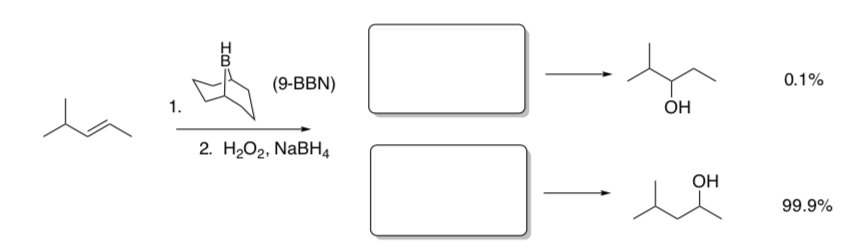
Conclusion: Sterically hindered borane reagents result in_______________.
Stereochemical control of hydroboration
- Circle sites of significant steric hindrance in the transition state of this hydroboration with a chiral borane reagent.
- Draw the products. Include stereochemistry!
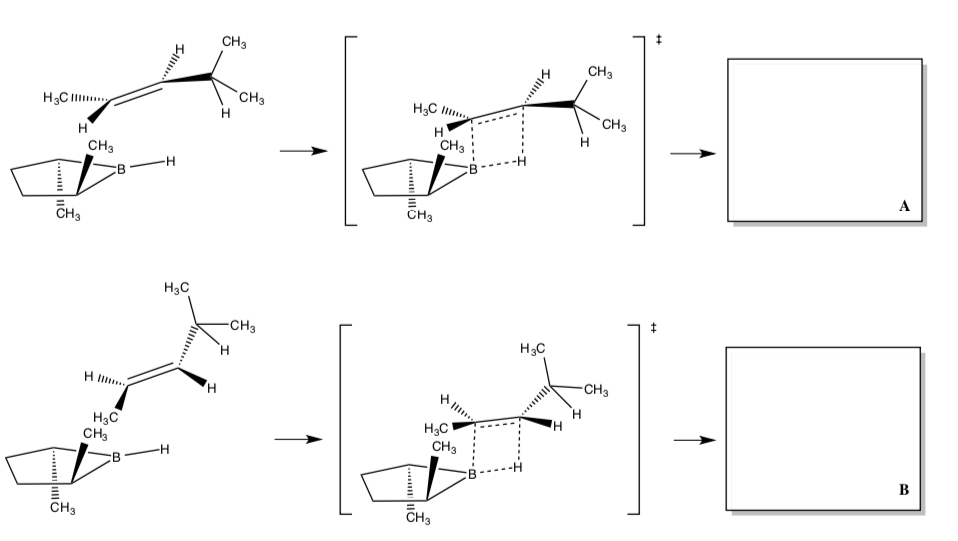
With the reaction above, only ONE stereoisomer is usually formed.
- Circle the favored product: A or B
- Explain why this boron reagent is enantioselective.
Singlet Carbenes
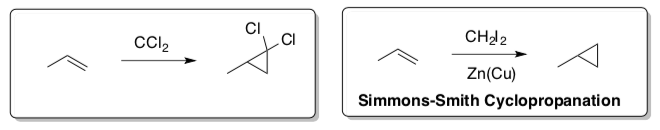
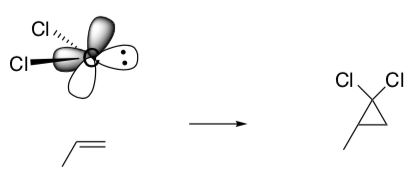
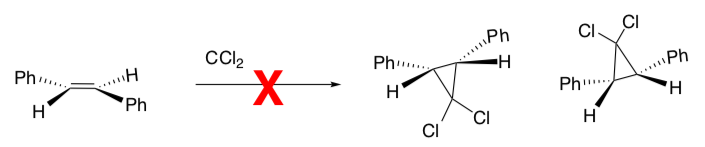
Carbenes are synthetically important because they represent a convenient way of preparing cyclopropanes.
CCl2 is a carbene, a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. 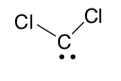
- Convince yourself that this carbene is neutral.
Valence electrons - # bonds – non-bonding electrons = formal charge
- While this carbene is neutral, it is not very stable. Why?
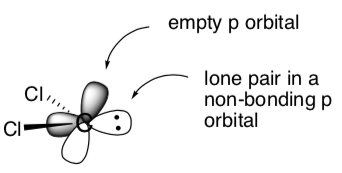
Here is a cartoon picture of the orbitals of this molecule.
- Label which orbital is the electrophile and which is the nucleophile.
- Draw the curved arrow mechanism for the concerted reaction of propene with the carbene, CCl2.
Epoxidation
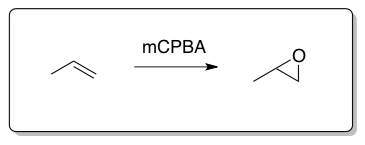
Epoxides can be prepared via the Williamson ether synthesis of a-halo alcohols. Direct epoxidation of alkenes can be carried out using percarboxylic acids.
Two common peracids used in epoxidations:
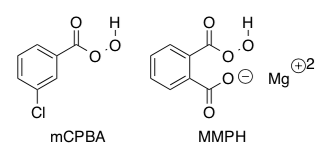
The mechanism of a peracid reacting with propene is shown below.
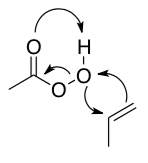
While this reaction is strangely concerted, it is sometimes easier to imagine if you break it down in steps:
- What is the nucleophile?
- What is the electrophile?
- What is the leaving group?
Stereochemical Implications of Concerted Mechanisms
When the carbene (or peracid) approaches the alkene, it forms a specific stereochemical product.
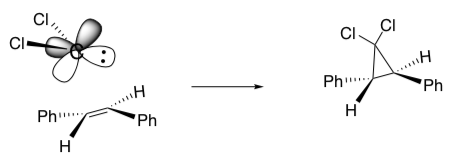
Of course, the carbene could also approach the other face of the planar alkene.
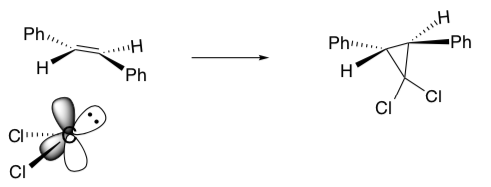
- However, the other two stereoisomers cannot be formed. Why?
- What are the products and stereochemical results of these concerted reactions?
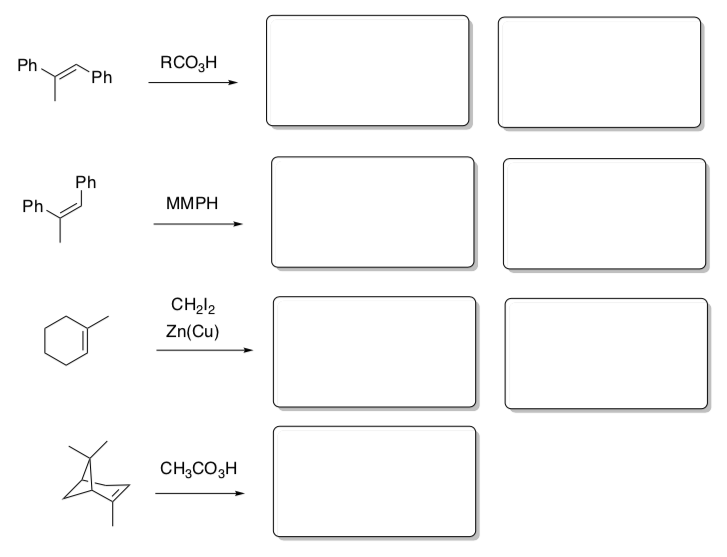
Concerted Electrophilic Additions
- Make Notecards. Make sure that you are comfortable with the variations.
You have three different mechanisms for Electrophilic Additions:
- Cationic Intermediate
- Cyclic Bromonium/Mercuronium Intermediate
- Concerted
- Draw the three different intermediates.
- Which type of intermediate allows for rearrangements?
Cationic | Cyclic Intermediate | Concerted | All | Neither
- Which type of intermediate leads to complete loss of stereocontrol?
Cationic | Cyclic Intermediate | Concerted | All | Neither
- With a carbocation intermediate, where does the nucleophilic substituent add?
More Substituted C | Less Substituted C | Anywhere it feels like it
- With the cyclic intermediate, where does the nucleophilic substituent add?
More Substituted C | Less Substituted C | Anywhere it feels like it
- With the concerted reaction, where does the nucleophilic substituent add?
More Substituted C | Less Substituted C | Anywhere it feels like it
- How does the geometric configuration of the starting alkene affect the stereochemistry of epoxidations or carbene reactions?
- How can you improve the control of stereo- and region-chemistry of hydroborations?
Application Problems
- Kinetics of Hydroboration
The kinetics of hydroboration have been studied extensively by H.C. Brown at Purdue. Brown looked at the rate of reaction of disiamylborane with cyclopentene, below.

- What is the probable rate law for the reaction?
To simplify Brown’s experiments a little, if the borane concentration is equal to the alkene concentration, a linear relationship should result from one of the following plots, based on the relations: ln[borane] = - kt + ln[borane]0 OR 1/[borane] = kt + 1/[borane]0.
- Which expression describes a first-order reaction? Put a box around it.
- Which expression describes a second order reaction? Circle it.
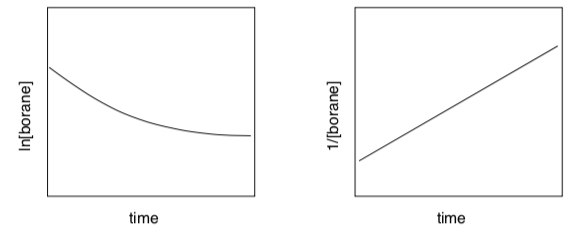
- For the hydroboration reaction, which plot is linear?
Experimental data looked something like this (simplified):
Dependence of Rate on Initial Reactant Concentrations in THF Solution [cyclopentene] (mol L-1) [disiamylborane] (mol L-1) Rate (mol L-1 s-1) 0.78 0.78 8.9 x 10-3 1.55 1.55 3.7 x 10-2 - What happened to the reactant concentrations? Same / doubled / quadrupled (circle)
- What happened to the rate? Same / doubled / quadrupled (circle)
- Write the rate law for the reaction, based on the data.
Brown also studied relative reaction rates for other alkenes, summarized in the table below.
Alkene k, L mol-1 s-1 1-pentene 1.07 1-octene 1.10 3-methyl-1-butene 0.58 3,3-dimethyl-1-butene 0.048 - Identify any trend in the data.
- Predict the products of the following reactions, assuming the same number of moles of borane and substrate are used in each case:
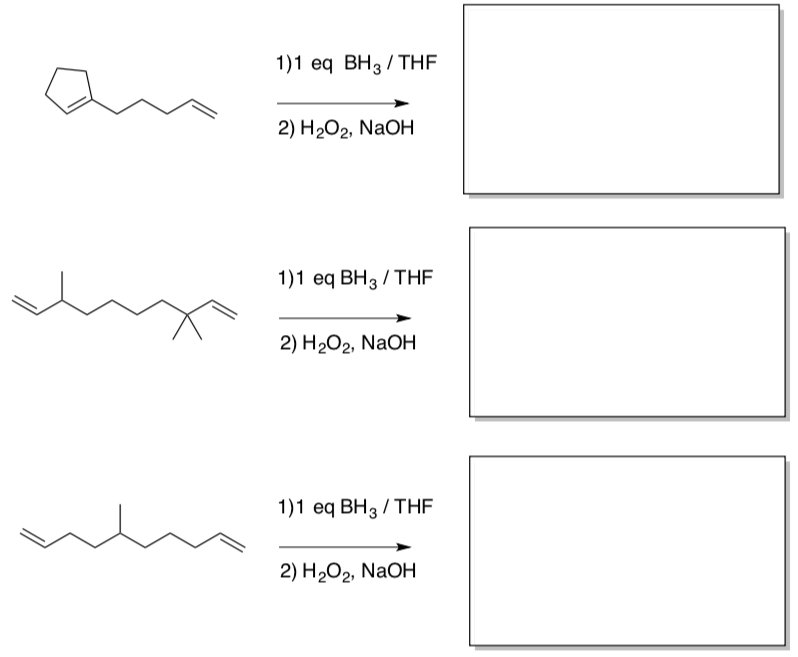
- Enzymatic Electrophilic Additions
S-adenosylmethionine
S-adenosylmethionine (SAM) is a great electrophile in biological systems. Du Vigneaud’s group in the 1940s determined SAM to be the source of many methyl groups in natural metabolites based on feeding isotopically labeled SAM to rats.
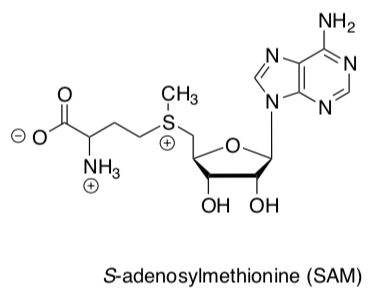
- Put a box around the electrophilic carbon.
- Circle the Leaving Group.
SAM is critical in the biosynthesis of epinephrine (adrenaline), a neurotransmitter.
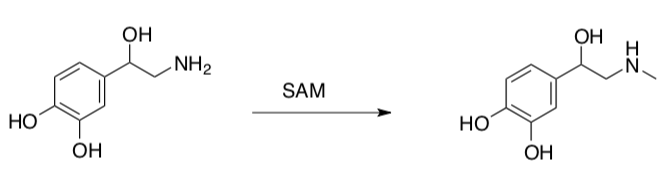
- Draw a mechanism for this reaction using curved arrows.
- Provide the name of this type of reaction.
- Why was the methyl added to the amine rather than the hydroxyl groups?
SAM is involved in the production of mycolic acids.
Mycolic acids, branched and hydroxylated long chain fatty acids (example shown below), are the fatty acids used in cell membranes of the Mycobacterium genus that causes human diseases such as tuberculosis and leprosy.
Boissier, Bardou, Guileet, Uttenweiller-Joseph, Daffe, Quemard and Mourey, J. Biol. Chem., 2006,281 (7), 4434-4445.
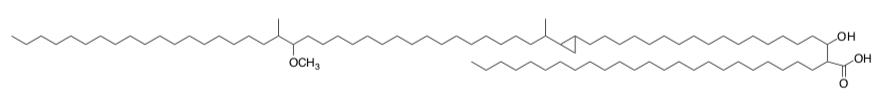
Branching on fatty acids is unusual and may be of crucial biological importance with respect to the permeability of the cell envelope. Studying the biosynthesis of the enzymes could help in finding new antitubercular drugs.
- Suggest a mechanism for this formation of hydroxyl methyl chain from an alkene:
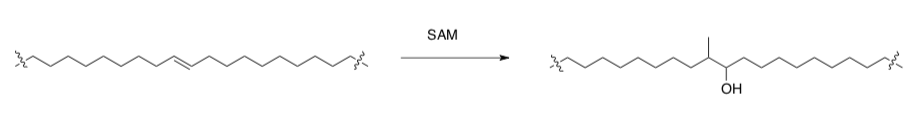
- Provide the name of this type of reaction.
Cyclopropanation: Two Possible Mechanisms
Squire Booker and his group has been interested in the biosynthesis of the cyclopropane group on the mycolic acid chains.
Iwig, Grippe, McIntyre and Booker, Biochemistry, 2004, 43, 13510-13524.

Two possible mechanisms were proposed.
- Draw arrows for this proposed mechanism.
- Fill in the missing intermediates.
- Circle the rds.
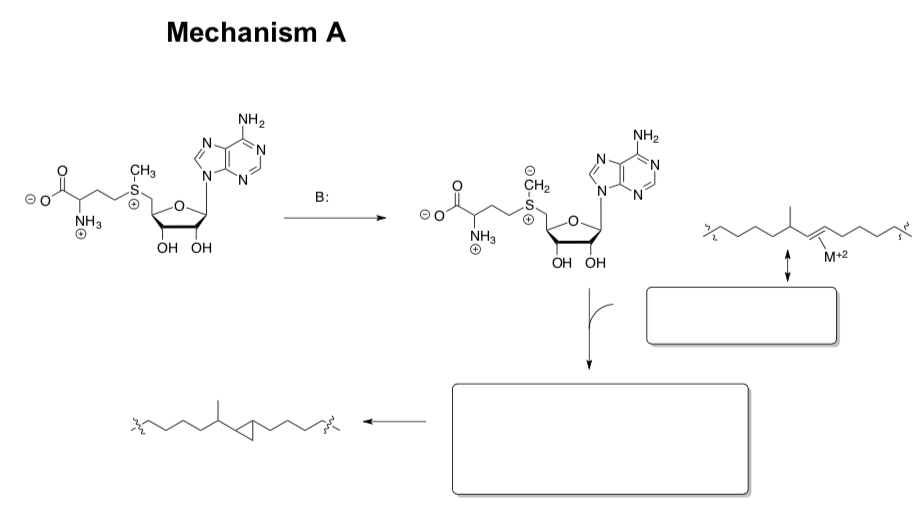
Enzymatic Electrophilic Additions (continued)
- Draw arrows for this proposed mechanism.
- Fill in the missing intermediate.
- Circle the rds.
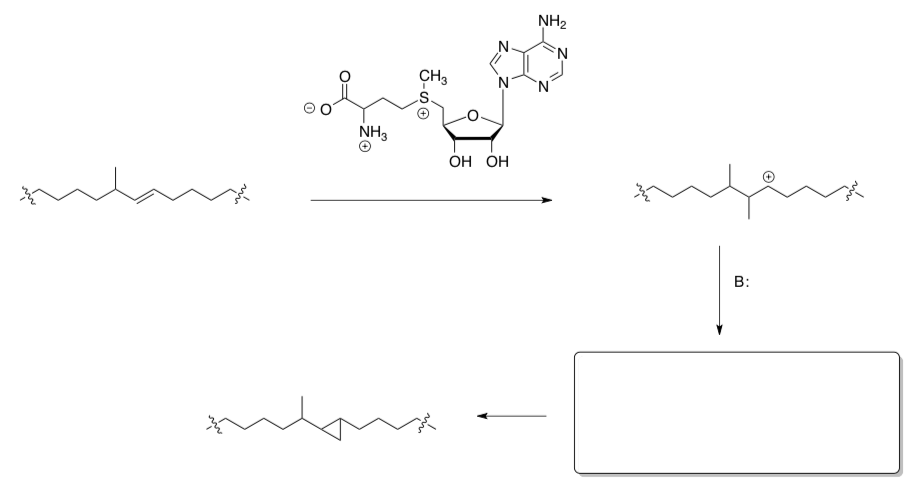
Enzymatic Electrophilic Additions (continued)
In a primary kinetic isotope study, the rate of the reaction involving C-H cleavage is compared to the rate involving C-D cleavage. If the bond is broken in the rate determining step, C-D cleavage is up to 7x slower than C-H cleavage.
Remember, the Michaelis-Menten relationship:
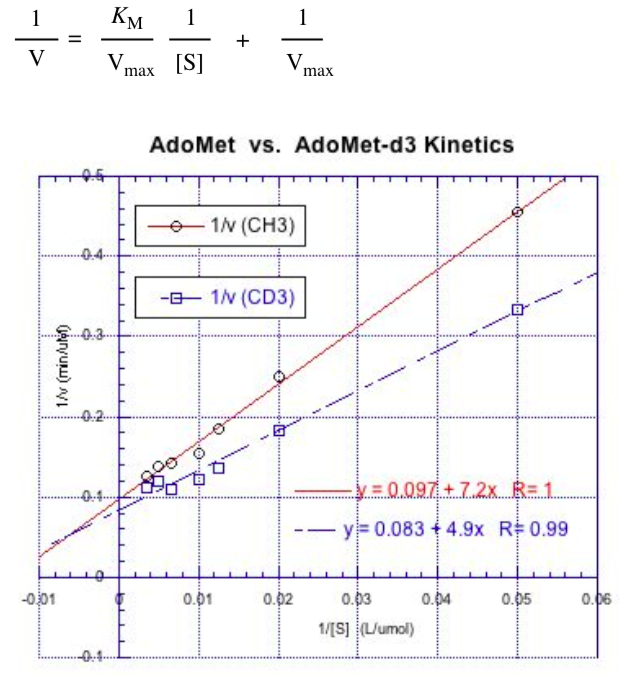
- Given [E] = 1 mM, determine kcat for
AdoMet(CH3): ____________
AdoMet(CD3): ____________
Enzymatic Electrophilic Additions (continued)
- How do these two kca values compare (circle one)?
kca(CH3)> kca(CD3) | kca(CH3)= kca(CD3) | kca(CH3)< kca(CD3)
- Is there a primary kinetic isotope effect in this case?
In SN2 reactions at methyl groups, CD3 exhibits an “inverse isotope effect” although the C-D bond is not broken. In this case, CD3 can undergo substitution slightly faster than CH3.
- Does there appear to be an SN2 reaction occurring? Explain.
- Which mechanism is more likely based on the kinetic data?
Mechanism A | Mechanism B
Catalytic Cycles with Electrophilic Addition
Suzuki
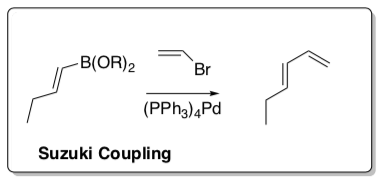
First published in 1979 by Akira Suzuki, the Suzuki reaction couples boronic acids (containing an organic substituent) to halides (2010 Nobel Prize).
- Provide a mechanism for the electrophilic addition reaction of catecholborane with this alkyne to form the starting material.
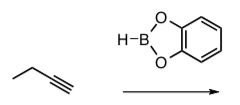
- Fill in the blanks on the following catalytic cycle for the Suzuki coupling.

Sharpless Epoxidation
Barry Sharpless received the Nobel Prize in 2001 for this stereoselective oxidation reaction.
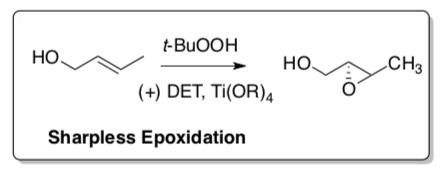
Katsuki and Sharpless, J. Am. Chem. Soc., 1980, 102, 5974-5976.
Ti(iOPr)4, optically active diethyl tartrate and t-butylhydroperoxide will stereoselectively epoxidize allylic alcohols.

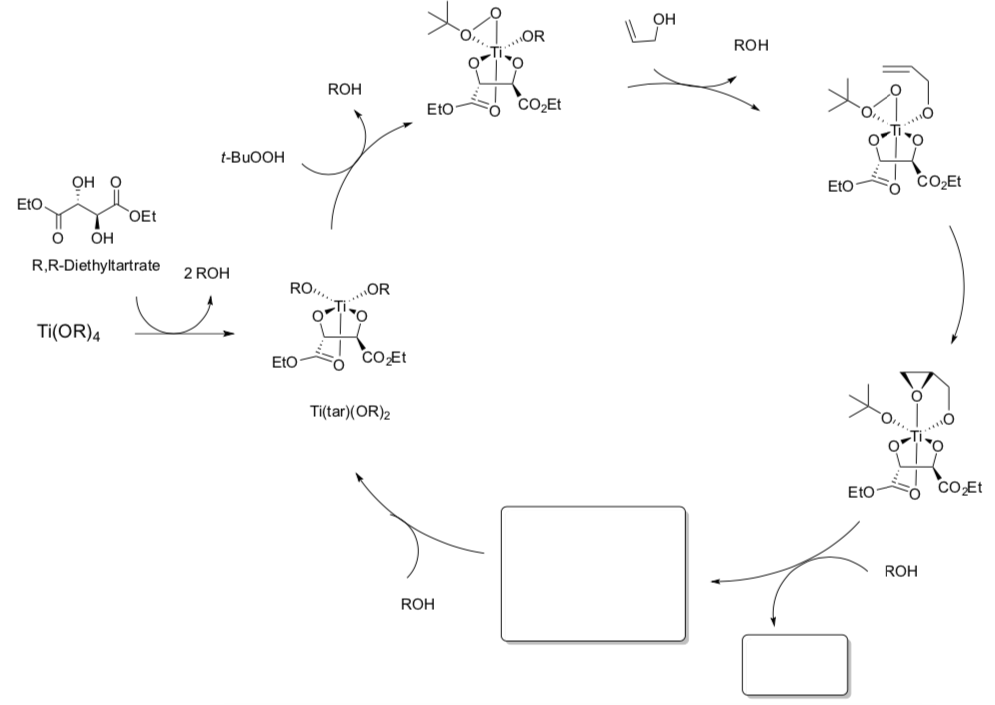
This is a simplified version of the catalytic cycle.
- Label each step with the type of reaction (association, dissociation, migratory insertion, 1,2-insertion, b-elimination, oxidative addition, reductive elimination).
- Draw the arrows for each step. (What’s happening with all the proton transfers??)
- Fill in the boxes.
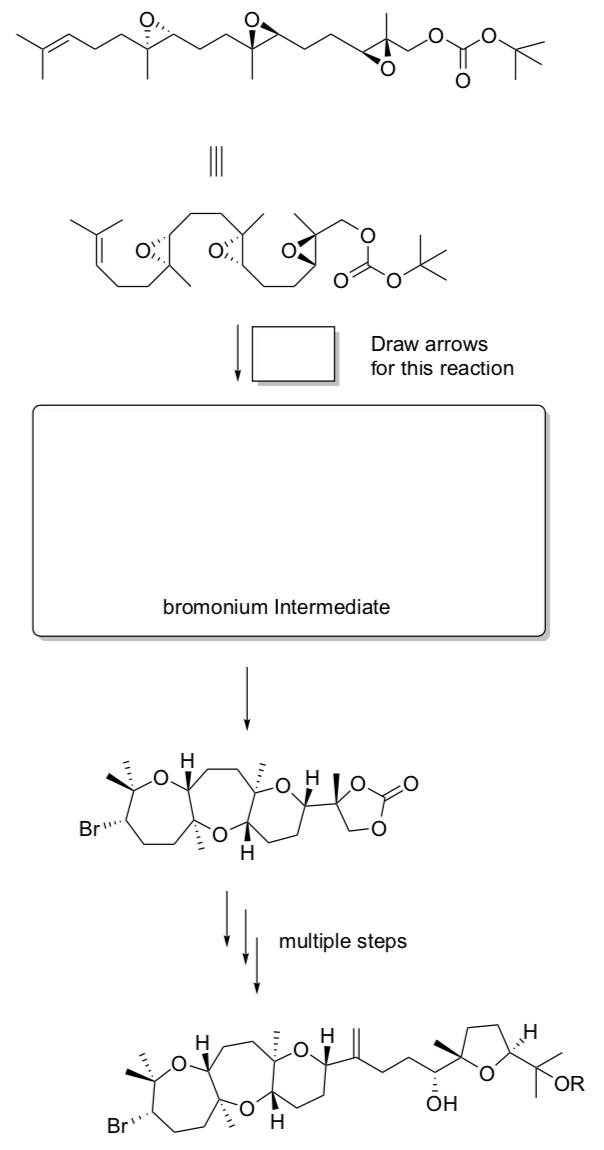
Application to Synthesis Problem:
Total Synthesis of ent-Dioxepandehydrothyrsiferol
Tanuwidjaja, Ng, and Jamison, J. Am. Chem. Soc., 2009, 131, 12084.
ent-Dioxepandehydrothyrsiferol is a natural compound isolated red alga.
- Fill in the missing reagents/products in the synthesis shown below:

The authors complete a critical step of this synthesis with one reagent.
- Provide this reagent and draw the arrows for the cyclization cascade mechanism.



