23: Electrophilic Aromatic Substitution (Worksheet)
- Page ID
- 148212
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
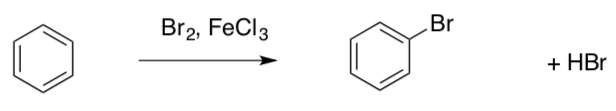
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile.

This is a two-step reaction mechanism with a carbocation intermediate.
First step: Aromatic rings (like alkenes) can also act as nucleophiles. The electrons of the ring are only attracted to very strong electrophiles.

- What mechanism does this resemble?
- What is the Nucleophile?
- What is the Electrophile?
- What is the role of the FeCl3?
Second step: The cationic intermediate rapidly undergoes elimination to reform the aromatic ring.

- What mechanism does this resemble?
- Why does this step occur so rapidly? What is the driving force?
MO Theory for Electrophilic Aromatic Substitutions
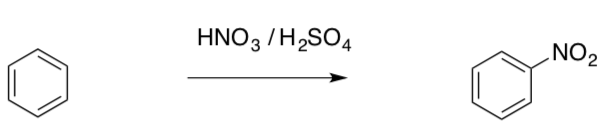
Alkenes can react directly with electrophiles such as halogens. However, with benzene, the electrophiles must first be activated.
- Suggest reasons for the difference in reactivity between an alkene and benzene.

- Draw all possible resonance structures of the carbocation formed in the mechanism above.
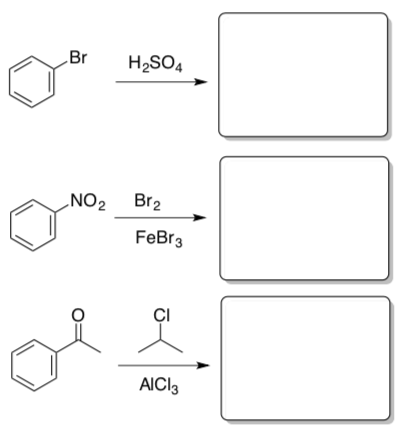
Practice Mechanisms
- Provide a mechanism for this reaction.

Sometimes, electrophiles that you have seen react with alkenes will react with aromatics, but only in the presence of a catalyst.
- Provide a mechanism for this reaction.
The same kinds of catalysts can promote other reactions.
- Provide a mechanism for this reaction.

Sometimes, things go wrong.
- Explain why this product provides multiple products.

Other electrophiles are less familiar. The following reaction proceeds through the nitrosyl cation, NO2+.
- Provide a mechanism for this reaction.
The following reaction is similar, and proceeds in highly concentrated sulfuric acid. Under those conditions, sulfur trioxide forms.
- Provide a mechanism for this reaction.

Practice Predicting Products
- Predict the products or reagents to complete the following reactions.

Electrophilic Aromatic Subsitution to substituted rings:
The presence of a substituent on an aromatic ring has two effects when an electrophilic aromatic substitution is attempted.
- Substituents affect the reactivity of the aromatic ring.
- Substituents affect the orientation of the reaction. Three possible disubstituted products can result (ortho, meta and para).
Activating/Deactivating Substituents
Below is a list of different substituents and experimental results from attempted electrophilic aromatic substitution.

- Is the aromatic ring an [ electrophile / nucleophile ] in these reactions?
- Which substituents ‘activate’ the ring (i.e. make it more reactive?
- Alkyl substituents: Activate OR Deactivate
- Halide substituents: Activate OR Deactivate
- OH, NH substituents: Activate OR Deactivate
- carbonyl groups: Activate OR Deactivate
- Nitro groups: Activate OR Deactivate
- In summary:
- Substituents with a lone pair on the atom next to the ring:
Activate OR Deactivate
- Substituents with a pi bond on the atom next to the ring:
Activate OR Deactivate
- Alkyl groups are electron donating through induced dipoles which:
Activate OR Deactivate
- Substituents with a lone pair on the atom next to the ring:
Regioselectivity of the Substitution
A previous substituent can also influence the position of 2nd electrophilic substitution.
For example:

| SUBSTITUENT | PRODUCTS (%) | ||
| ORTHO | META | PARA | |
| CH3 | 63 | 3 | 34 |
| CN | 17 | 81 | 2 |
| NO2 | 7 | 91 | 2 |
| CHO | 19 | 72 | 9 |
| CO2CH3 | 18 | 76 | 6 |
| Cl | 35 | 1 | 64 |
| F | 23 | 1 | 76 |
| Br | 43 | 1 | 54 |
| OH | 50 | 0 | 50 |
| NH2 | 28 | 2 | 70 |
- Using this regioselectivity data, develop a set of rules to predict products:
- Alkyl substituents: o,p directors OR meta directors
- Halide substituents: o,p directors OR meta directors
- OH, NH substituents: o,p directors OR meta directors
- carbonyl groups: o,p directors OR meta directors
- Nitro groups: o,p directors OR meta directors
- Fill in the blanks:
Electron donating groups are usually _______________.
Electron withdrawing groups are usually _______________.
Halides are the exception - they are electron withdrawing and ______________.
Resonance of Cationic Intermediates
Substituents with a lone pair on the atom attached to the ring (-OH, -NH2, OCH3, etc) can donate that pair into the extended π conjugated system to increase the nucleophilicity of certain positions on the ring.
- Draw resonance structures of this starting compound.
- Identify which carbons have increased electron density.
- Use these structures to explain why these substituents are typically o,p directors.

Substituents with p bond to the atom attached to the ring (-C=O, -NO2, etc) can withdraw electron density from the aromatic π conjugated system to decrease the nucleophilicity of certain positions on the ring.
- Draw resonance structures of this starting compound.
- Identify which carbons have increased electron density.
- Use these structures to explain why these substituents are typically m directors.

Halides are usually o, p directors.
- Draw resonance structures of bromobenzene.
- Explain why these substituents are o,p directors using resonance structures.
Practice Synthesis with Directing Effects
- Show the major products of these reactions.

Summary of Electrophilic Aromatic Substitutions
Types of reactions:
There are limited electrophiles that are strong enough to react with an aromatic ring.
- List the 5 electrophiles that can be added to an aromatic ring with EAS:
- Draw a generic electrophilic aromatic substitution reaction.
Reactivity
- Activated aromatic rings are very ( likely / unlikely ) to undergo another substitution.
- Deactivated aromatic rings are very ( likely / unlikely ) to undergo another substitution.
- When the aromatic ring is substituted, ___________ groups are activating and and __________ groups are deactivating.
- Activating groups have this structural feature:_______________.
- Deactivating groups have this structural feature:_______________.
Directing Effects
A previous substituent influences the position of 2nd substitution.
Electron donating groups are _______________ directors.
Electron withdrawing groups are ___________ directors.
Halides are the exception - they are electron withdrawing BUT they are _________directors.
Application Problems
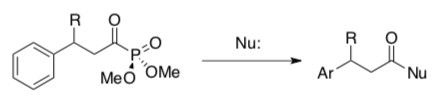
- Evans Chiral Electrophilic Aromatic Substitution.
Evans (Harvard) developed a chiral electrophilic aromatic substitution reaction.
Evans then developed a nucleophilic addition reaction to remove the phosphonate ester.
Evan’s chiral electrophilic aromatic substitution methodology was used in the synthesis of heliotridane.
- Circle the nucleophile and the electrophile in the EAS reaction below.

Using the Evan’s Lewis Acid, the reaction yields a chiral product.

- What is the role of Evan’s catalyst (Catalyst 1)? Show an arrow.
- Why does this catalyst provide a chiral product?
- Provide a mechanism for this reaction.
- Using Evan’s chiral methodology, predict products in this sequence.
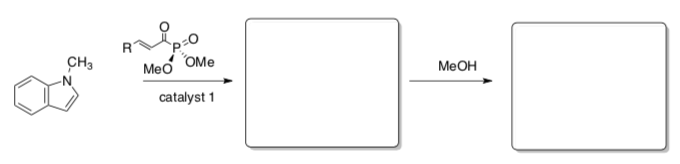
- The first step in the synthesis of heliotridane involves an electrophilic addition to pyrrole. Use the HOMO of pyrrole to explain why addition occurs near the NH.

- Complete the missing steps in the synthesis of heliotridane.

- Circle the nucleophile and the electrophile in the EAS reaction below.
- Houamine B Synthesis
Houamine A and B are potential antitumour agents with activity against human colon cancer cells HT-29 and mice endothelial MS-cells, respectively.
Trauner, Organic Letters 2006, 8, 23-25

- Fill in the blanks in the partial synthesis of houamine B.

- Propose mechanisms for the acid-catalysed conversions indicated midway through the synthesis.
- Given the following information about the fate of alcohols in acidic lithium perchlorate solution, propose a mechanism for the final step in the synthesis.

- An alternate approach involves the following reaction. Propose a mechanism.

- Fill in the blanks in the partial synthesis of houamine B.
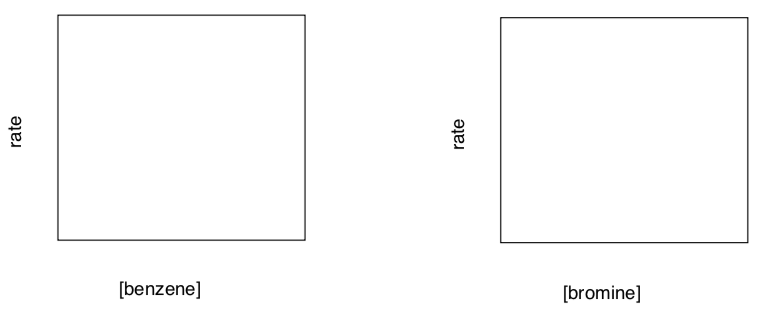
- Kinetics of Aromatic Bromination
A number of studies during the 1950’s revealed that the kinetics of aromatic bromination is complicated by multiple bromine species in equilibrium. The non-catalyzed reaction in aqueous solution, although much slower, is more straightforward (Felicia Gaskin & Ernst Berliner, 1966).
Dependence of Rate on Initial Reactant Concentrations in Aqueous Solution [benzene] (mol L-1) [bromine] (mol L-1) Rate d[PhBr]/dt (mol L-1 s-1) 0.007541 0.0018 1.95 x 10-8 0.01092 0.0018 2.61 x 10-8 0.01092 0.0037 5.65 x 10-8 0.01411 0.0018 3.45 x 10-8 - Determine the order in benzene.
- Determine the order in bromine.
- Write the rate law for the reaction.
- Fill in the following graphs (straight line with positive slope, negative slope, curve, etc).
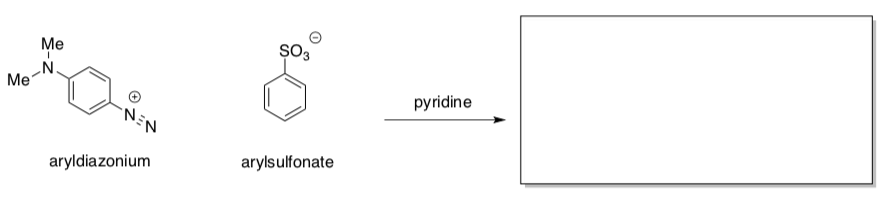
- Kinetics of Aza Dye Formation
Diazonium ions (Ar-N2+, Ar = aromatic) are very good electrophiles; a number of commercially important aza dyes are produced via reactions like the following one. The pyridine acts as a base in the reaction.
- Predict the product of the reaction.
The following is an example of the reaction; some virtual rate data is shown below the reaction.

- Write a rate law for the reaction.
When an experiment is run with isotopically-labeled compounds, it is frequently observed that carbon-deuterium (2H, symbol D) bonds break up to 7x more slowly than carbon-protium (1H) bonds. In the reaction above, however, the rate constants are about equal: kH/kD = 1.
- Why is there no isotope effect on this rate?
The following is an example of a very similar reaction; some virtual rate data is shown below the reaction.
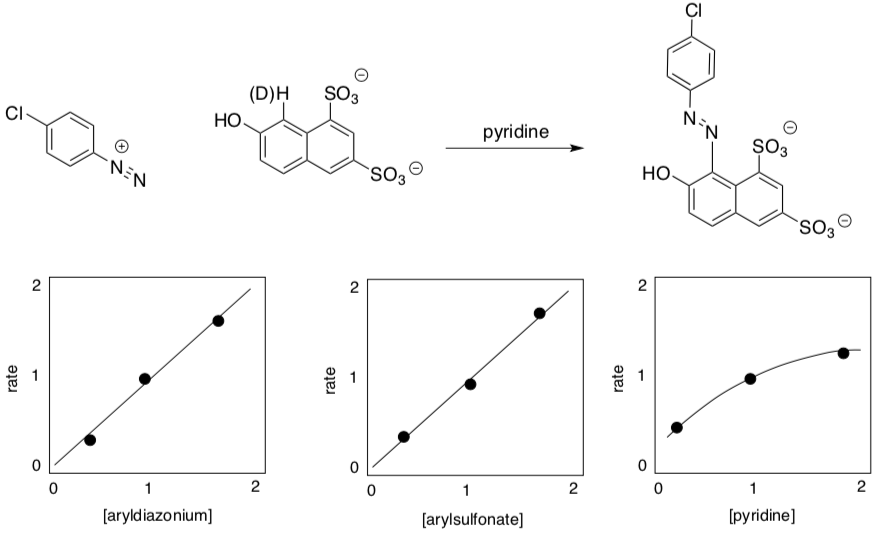
- Does the rate depend on pyridine concentration?
- Is the relationship linear (i.e. simply first order in pyridine)?
- Predict the product of the reaction.
Multi-step Synthesis
Useful Aromatic Ring Modifications:
Here are some functional group modification reactions that you should be familiar with as they are useful in the synthesis of aromatic compounds.
Note
These reactions should all be added to your note cards.

Short Retrosyntheses
- Propose syntheses for these compounds from benzene. effects.
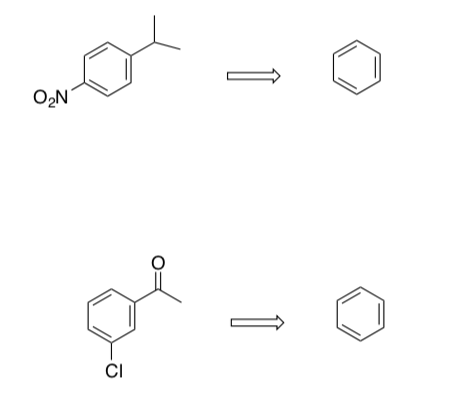

- Challenge: Propose a synthesis for 3-chlorotoluene from benzene. Keep in mind two factors: 1) Formyl chloride does not exist. 2) The yields on methylation using Friedel-Crafts alkylation are very low so this is not the optimal route.
Synthesis Race
Your instructor will put a synthetic target on the board. The first group that proposes a short, work-able synthesis will receive one extra credit point.
Rules:
- Only one functional group per starting material/reagent.
- Minimum of six carbons per starting material/reagent.
- Protecting groups must be used as necessary.
- Consider the order of steps!
Additional Problems:
- Starting from benzene, devise three different routes to ethylbenzene (not just 3 different Friedel-Crafts reagents).
- These three compounds do not undergo Friedel/Crafts Reactions. Why?
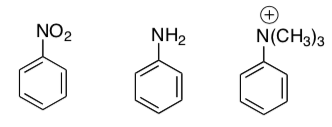
- Devise a synthesis of 3-chloro-ethylbenzene from benzene.


