20: OM Catalysis
- Page ID
- 144252
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCReview: Organometallic Reaction Steps
In organometallic catalysis, catalytic mechanisms are often made of 7 types of steps: association, dissociation, 1,1-insertion, 1,2-insertion, b-elimination, oxidative addition and reductive elimination.
Let’s review the steps that we have covered previously.
Review*
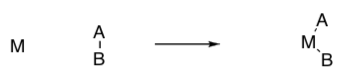
- Draw the product of this association reaction.
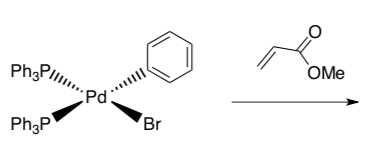
- Draw the product of this dissociation reaction.
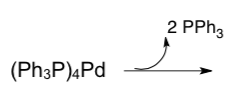
- Draw the product of this 1,1 insertion (also called migratory insertion).
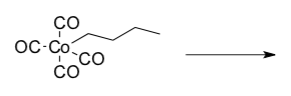
- Draw the product of this 1,2 insertion.
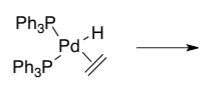
- Draw the product of this β-elimination.
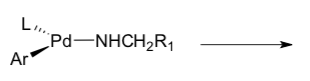
*Answers for this Review of OM Reactions section are available on Canvas
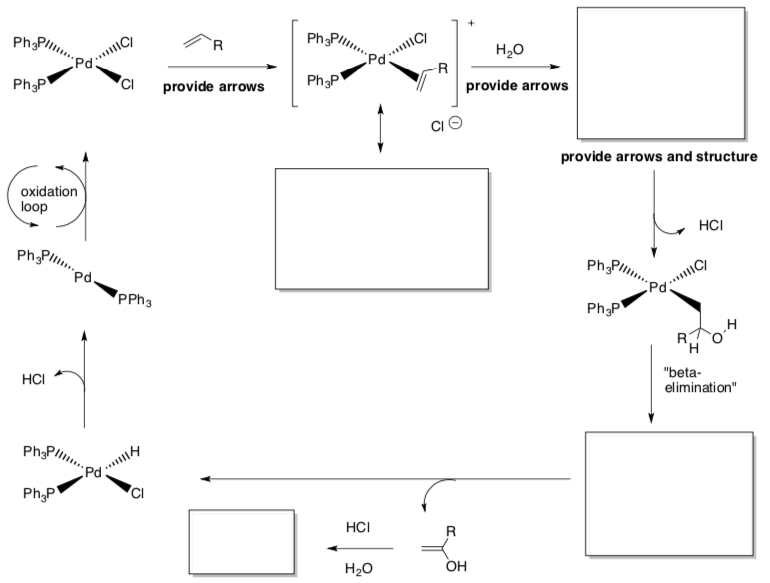
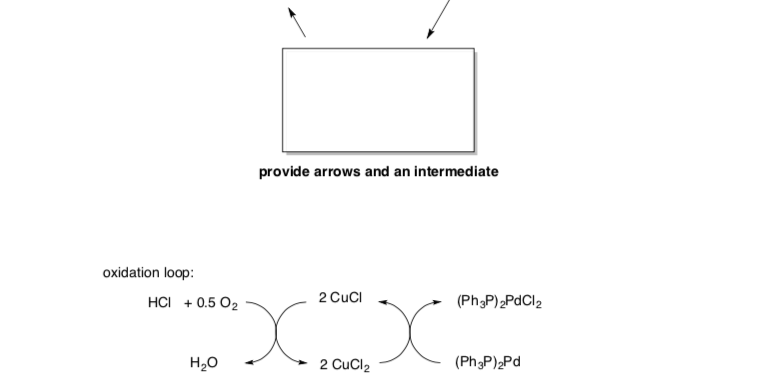
Catalytic Cycles
Catalytic Cycle is a term for a multistep reaction mechanism that involves acatalyst. Since catalysts are regenerated, catalytic cycles are usually written as a sequence of chemical reactions in the form of a loop.
- Fill in the blanks on the cycle below:
- What happened to the oxidation state on the metal in the last two steps?
Oxidative Addition and Reductive Elimination
Oxidative addition is the addition of a bond to a metal center.
Reductive elimination is the reverse reaction.
Why is the forward reaction called an oxidative addition?
- What is the oxidation state on the metal in the reactants and the products of this reaction?
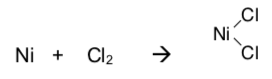
- Explain why this is an oxidation (remember: lose e- oxidize, gain reduce).
- On the previous page, label the oxidative addition in the cycle.
- On the previous page, label the reductive elimination in the cycle.
Oxidative Addition Mechanisms
There are two possible mechanisms.
- The metal can donate a pair of electrons to one of the atoms, displacing the other atom as an ion. This ion recombines with the metal.
- The addition can be concerted.
1. Stepwise Polar Addition
- The polar oxidative addition mechanism is similar to an SN2 mechanism. BUT in this reaction, the metal is the nucleophile. That should seem very strange. Why?
- To do an SN2 on a substrate, what structural characteristics does the substrate need?
- Show the mechanism with arrows for addition of a) HCl and b) CH3CH2Br to a metal.
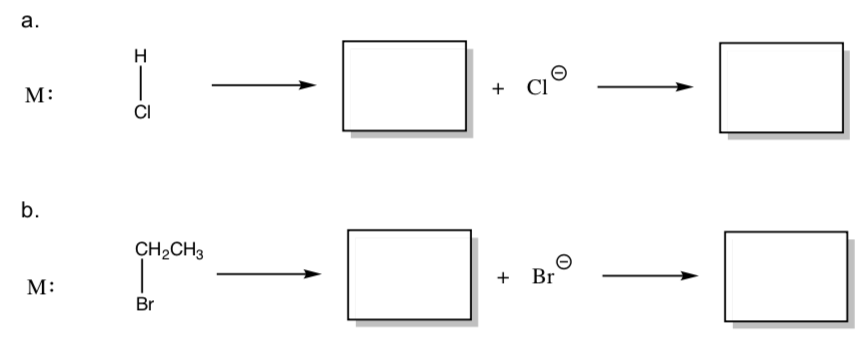
- Polar Oxidative Additions are much faster in polar solvents. for this phenomenon.
- The addition of H2 to nickel does not proceed through this mechanism. Explain why.
2. Concerted Oxidative Addition
The concerted mechanism (all bond-making and bond-breaking steps occur at the same time) occurs when there is no leaving group on an sp3 hybridized atom.
- Draw a curved arrow mechanism for a concerted addition of H2 to a metal atom.
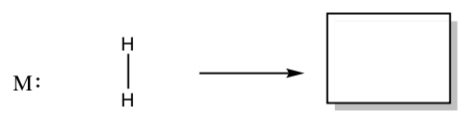
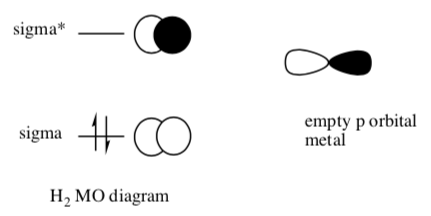
Understanding how the concerted mechanism works may require some MO theory.
- Show the overlap of an occupied molecular orbital on hydrogen (H2) and its interaction with an unoccupied p orbital on nickel.
- Draw an occupied metal d orbital and its interaction with an empty molecular orbital on hydrogen (H2).
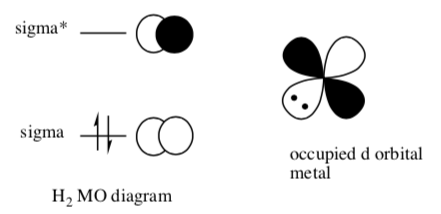
- Explain how these interactions combine to break the H-H bond.
Practice Problems
- Choose the most likely mechanism for the following cases (one-step or two- step).
- Explain why you chose this mechanism for these cases.
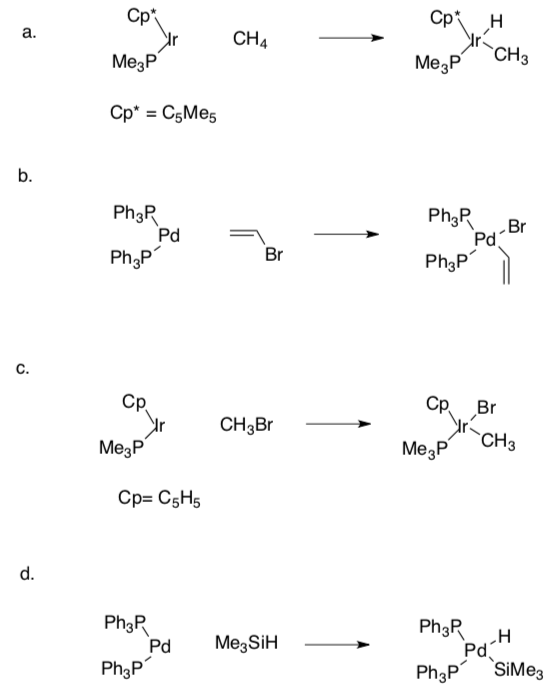
Summary of Oxidative Addition and Reductive Elimination
Oxidative Addition
- Show an example of an oxidative addition reaction step.
- What happens to the charge on the metal in an oxidative addition?
- What are the two possible mechanisms for oxidative addition?
- How can you predict which mechanism will proceed?
Reductive Elimination
- Show an example of a reductive elimination reaction step.
- What happens to the charge on the metal in a reductive elimination?
Oxidative Addition Mechanism Application Problem
- How do we know the order of steps for polar oxidative addition?
While we presented that polar oxidation proceeds like an SN2 mechanism, one could also propose a reasonable alternate pathway:
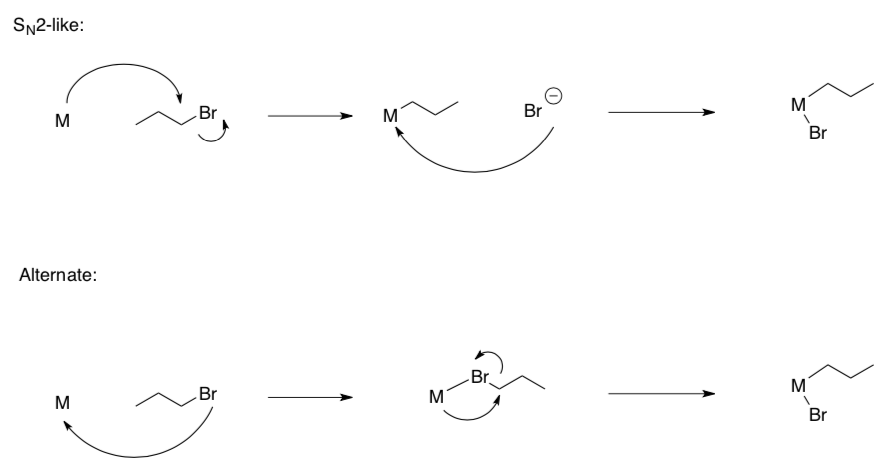
Mechanistic Data:
- This reaction results in the alkyl and halide being trans to each other.
- Does this data suggest the SN2-like OR Alternate pathway?
- Why?
- This reaction proceeds faster in polar solvents.
- Does this data suggest the SN2-like OR Alternate pathway?
- Why?
- This reaction results in the alkyl and halide being trans to each other.
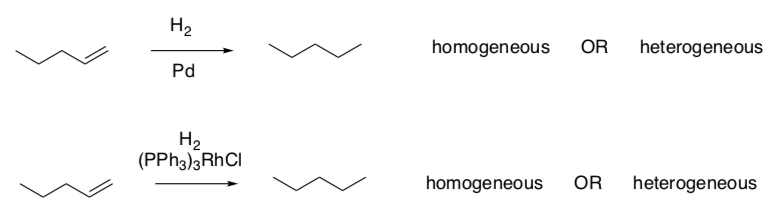
Hydrogenation
Hydrogenation is used in the food industry to make a large variety of manufactured goods, like Crisco or margarine from liquid oils. It is also used in the fuel industry.
In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene to form an alkane.
The catalyst can be a homogeneous catalyst or a heterogeneous catalyst.
- In the following two examples, determine which type of catalyst is being employed.
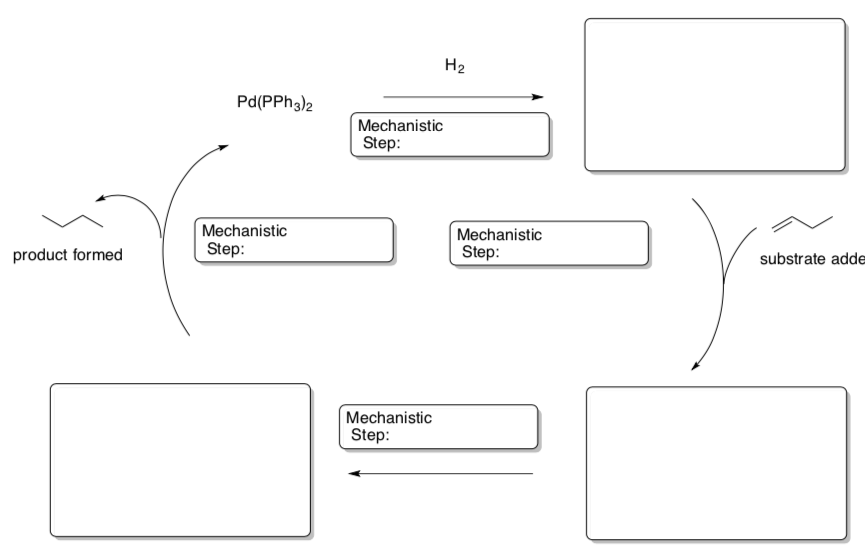
Hydrogenation Mechanisms
Homogeneous Catalytic Cycle
- Fill in the blanks on this cycle using a Pd(PPh3)2 catalyst.
- Label each step as: binding, reductive elimination, oxidative addition, or 1,2-insertion.
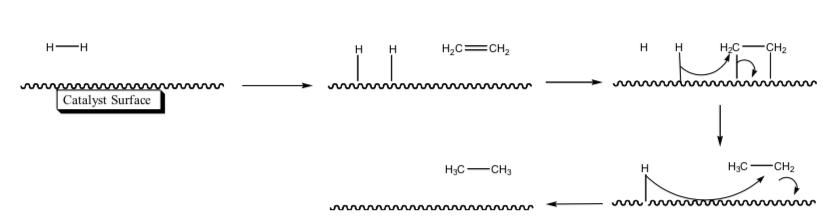
Heterogeneous Catalytic Hydrogenation
On solid metal catalysts, the accepted mechanism is the Horiuti-Polanyi mechanism.
Some of the most common metal catalysts are Pt, Pd, and Ni. These are usually placed on a highly dispersed powder support with large surface area (carbon or alumina)
Hydrogenation Catalyst Strengths
The rate of reduction is based on the strength of the catalyst.
| Catalyst | Alkenes |
| H2/PtO2, High Pressure | alkenes and aromatic |
| Crabtree’s Catalyst: (COD)Ir(PCy3)py]PF6 | tetra-substituted & lower |
| H2/Pd | tri-substituted & lower |
| Wilkinson’s Catalyst: (PPh3)3RhCl | di-substituted & lower |
| Lindlar’s Catalyst: Pd, quinoline, BaCO3 | alkynes only |
Stability of the Alkenes
The rate of reduction is partially based on the availability of the π-bond electrons and the stability of the alkene.
-> The more conjugated, the more stable.
-> The more carbons substituting around the pi bond, the more stable.
- Rank the following π-bonds in order of π-electron availability and substitution (1 = most reactive).
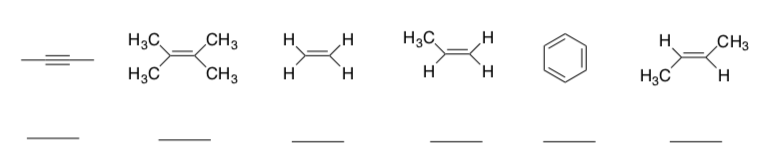
- Based on your answer to the above question, which catalyst could selectively hydrogenate an alkyne to an alkene.
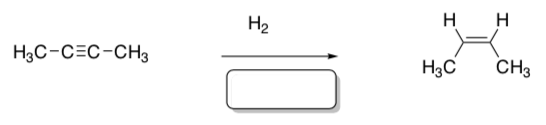
- Which catalyst(s) could perform this hydrogenation?
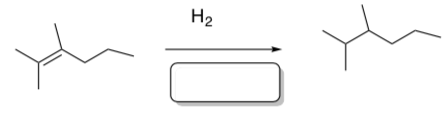
Stereochemistry of Hydrogenations
Hydrogenation results in syn addition of the hydrogens.
- Based on the mechanisms provided, explain why only syn addition is seen.
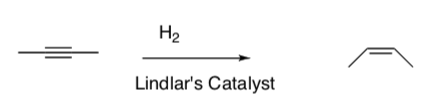
- Based on the mechanisms provided, explain the following observation.
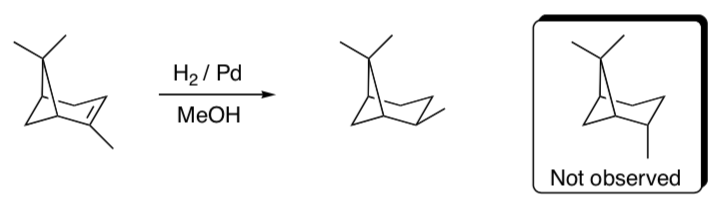
Summary of Hydrogenation Reactions
- Predict the products of these reactions
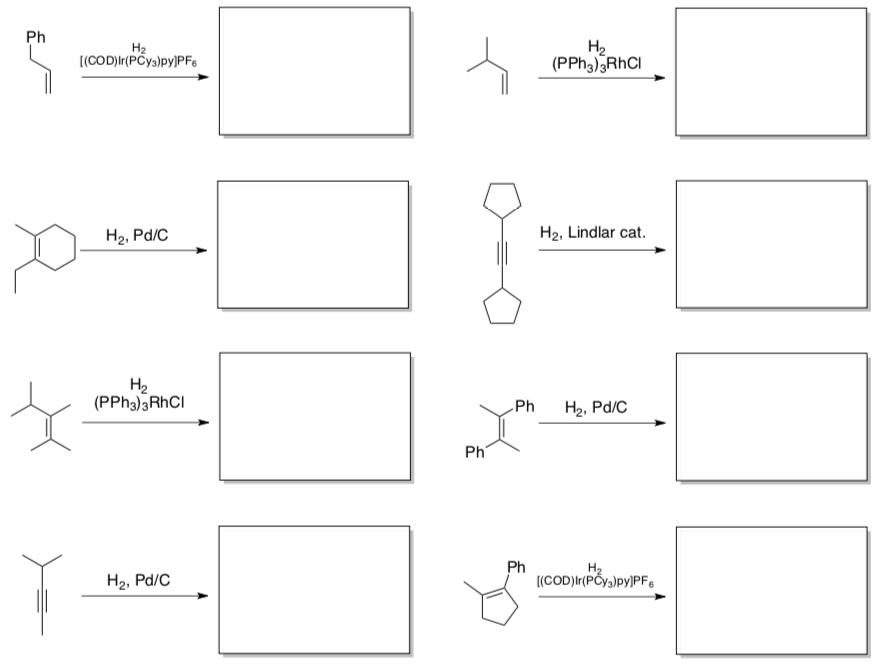
- Summarize the selectivity of Wilkinson’s catalyst, Lindlar’s catalyst and Crabtree’s catalyst.
- What is the typical stereochemistry resulting from a catalytic hydrogenation?
Application Problems
- Compare the selectivity of these two catalysts.
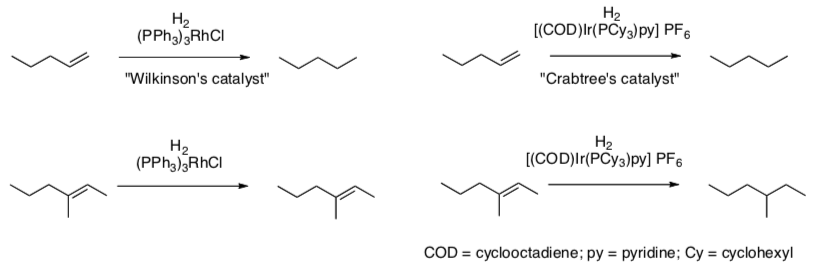
- Which catalyst is stronger?
Crabtree’s Catalyst OR Wilkinson’s Catalyst
- Draw both catalysts.
- Draw both catalysts after the H2 is added.
- Provide the geometry and electron count for each catalyst (before and after H2 addition).
- COD is a sacrificial ligand. What happens to the COD on Crabtree's catalyst with the hydride? Draw the product of this reaction. Now what is the geometry and electron count?
- Explain the difference in selectivity between the two hydrogenations catalysts.
- Which catalyst is stronger?
Classic Organometallic Transformations
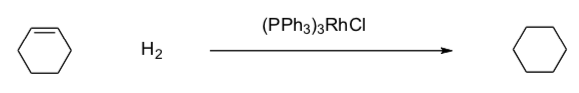
Review: Alkene Hydrogenation
- Draw a catalytic cycle for the following reaction.
(Hint: You have already done this cycle)
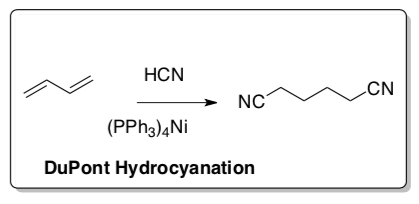
DuPont Hydrocyanation (in nylon synthesis)
- Count electrons for each metal complex in the cycle shown below.
- Label each step with the type of reaction (association, dissociation, migratory insertion, 1,2-insertion, b-elimination, oxidative addition, reductive elimination).
- Draw the arrows for each step.
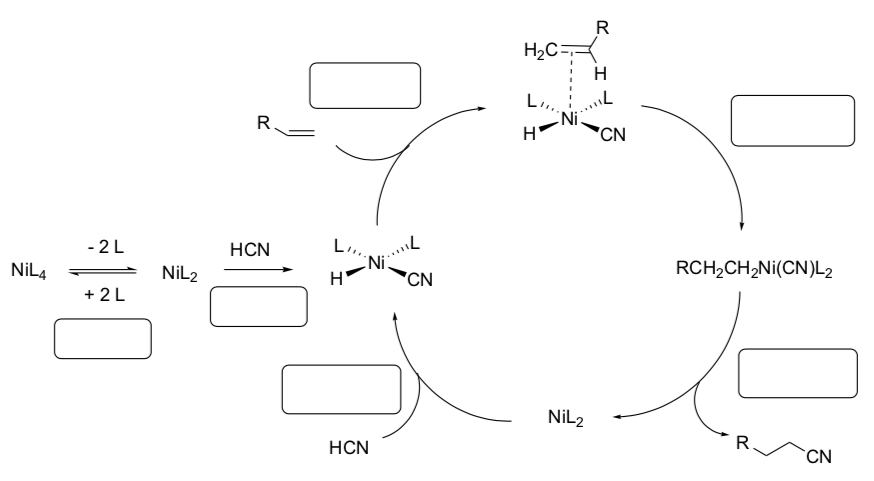
- How many turns on the cycle to make the precursor for Nylon-6,6 (shown in the notecard above)?
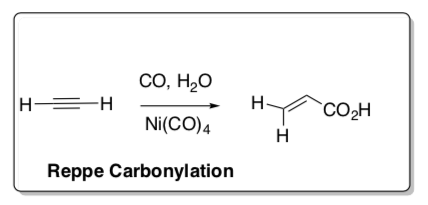
Reppe Carbonylation
(Very similar to DuPont Hydrocyanation)
- Fill in the missing metal complexes.
- Label each step with the type of reaction (association, dissociation, migratory insertion, 1,2-insertion, b-elimination, oxidative addition, reductive elimination).
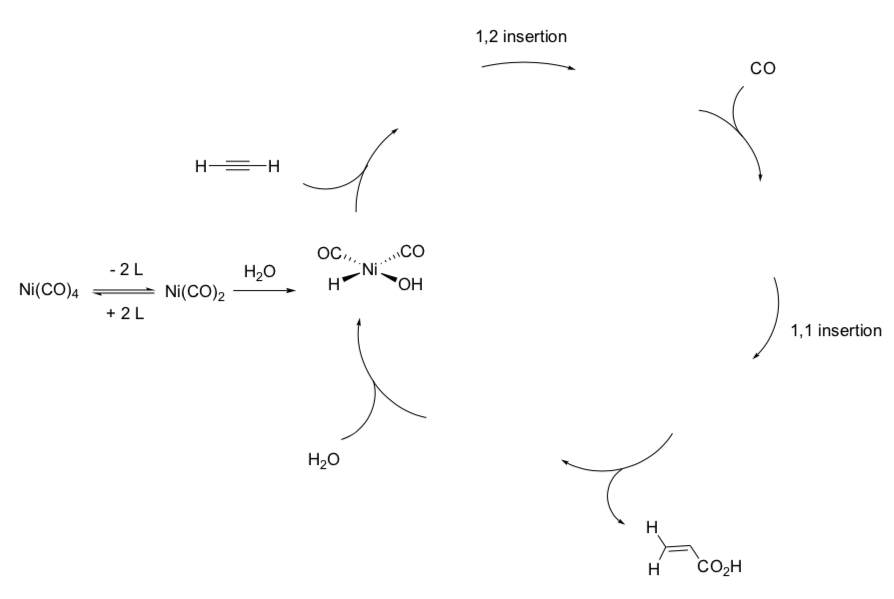
- Predict the product if ethanol (CH3CH2OH) is used rather than H2O.
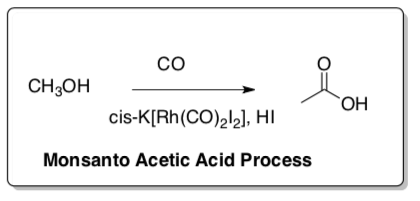
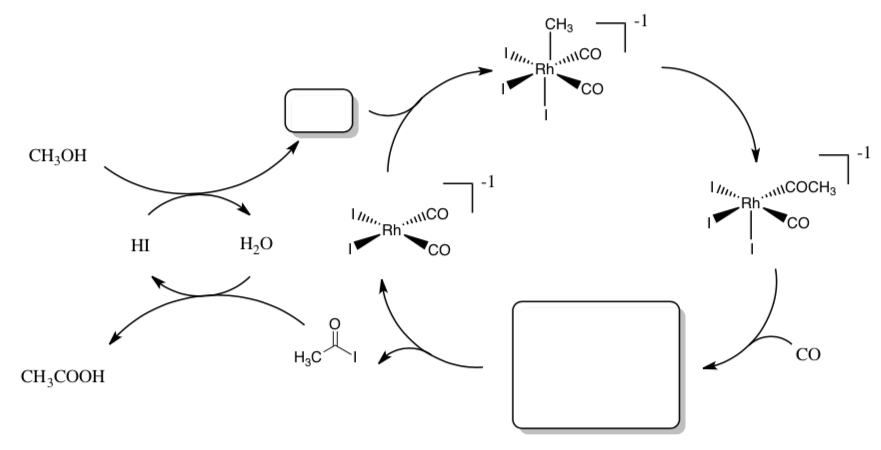
Monsanto Acetic Acid Process
Efforts to establish organic syntheses on the basis of C1 building blocks from coal resources include the Monsanto Acetic Acid Process. This cycle involves homogeneous catalysis: carbonylation of methanol.
- Count electrons for each metal complex in the cycle shown below.
- Label each step with the type of reaction (association, dissociation, migratory insertion, 1,2-insertion, β-elimination, oxidative addition, reductive elimination).
- Draw the arrows for each step.
- Fill in missing products.
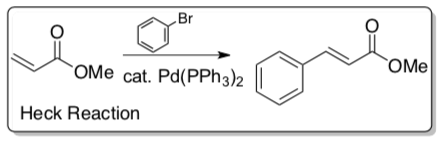
Heck Reaction
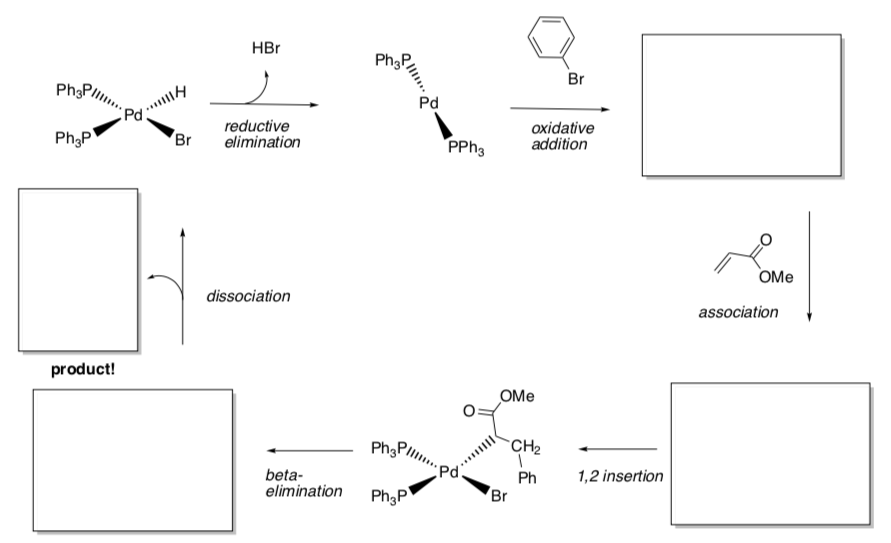
- Draw the arrows for each step.
- Fill in missing products.
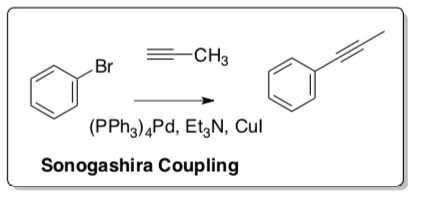
Sonogashira Coupling
Sonogashira (1975) developed a method to form a carbon-carbon bond between a terminal alkyne and an aryl or vinyl halide.
- Count electrons for each metal complex in the cycle shown below.
- Label each step with the type of reaction (association, dissociation, migratory insertion, 1,2-insertion, b-elimination, oxidative addition, reductive elimination).
- Draw the arrows for each step.
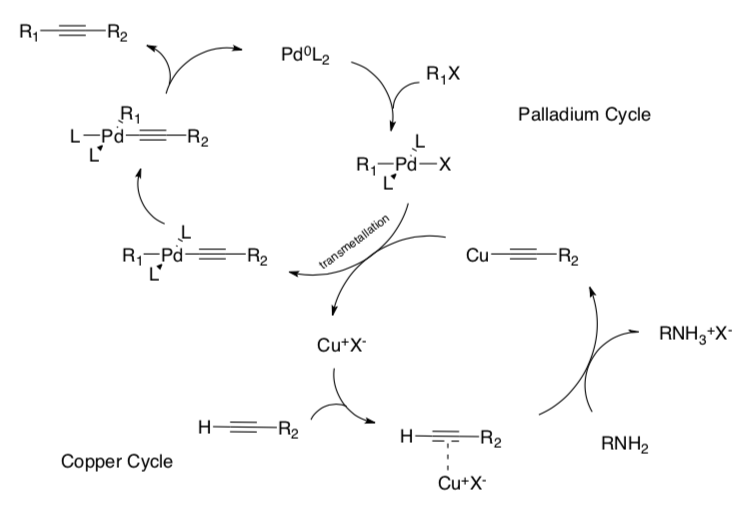
- Label the cis-trans isomerization step.
- Why did the square planar Pd complex have to undergo isomerization for this cycle?
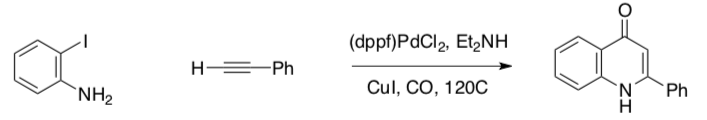
Sonogashira Application
Sonogashira reaction also has a carbonylation variation. The following example shows the synthesis of a pyridone in which the carbonylation and cyclization occur in tandem (Kalinin, Tet. Lett., 1992, 33, 373).
- Propose a cycle for this process.
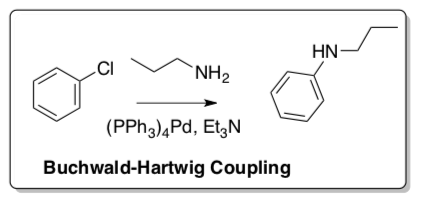
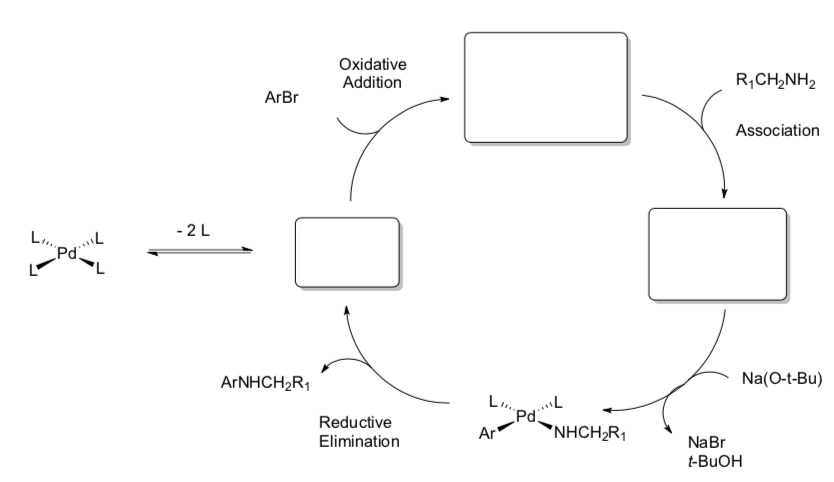
Buchwald-Hartwig Coupling
- Count electrons for each metal complex in the cycle shown below.
- Fill in the missing metal complexes.
- Draw the arrows for each step.
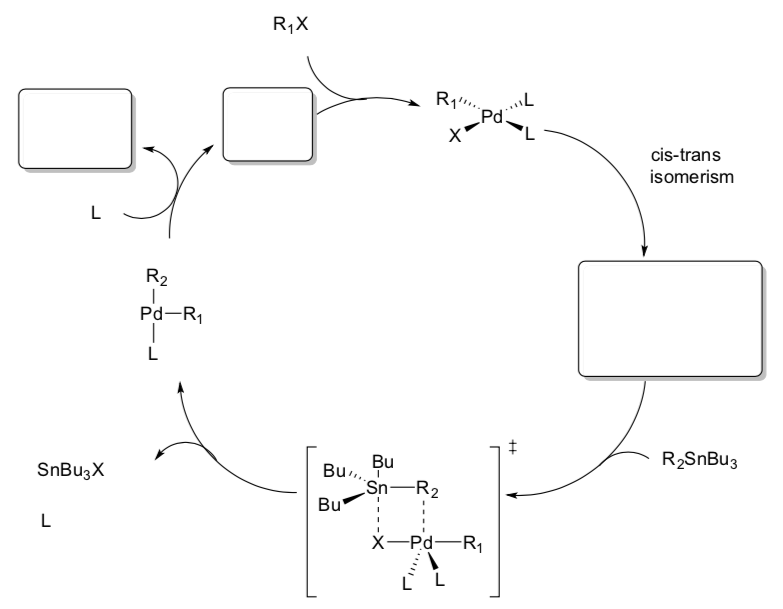
Stille Coupling
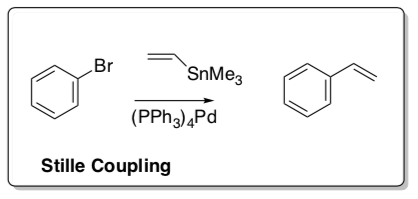
- Count electrons for each metal complex in the cycle shown below.
- Fill in the missing metal complexes.
- Draw the arrows for each step.
Stille Coupling (Carbonylative Variation)
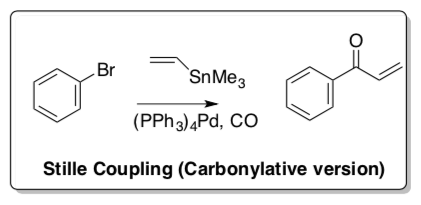
Like many of these reactions, there is a carbonylative version of the Stille Coupling.
- Propose a catalytic cycle for the carbonylative version shown above.
Wacker Reaction
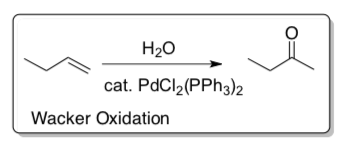
- Draw the arrows for each step.
- Fill in missing products.
- Draw the overview reaction.
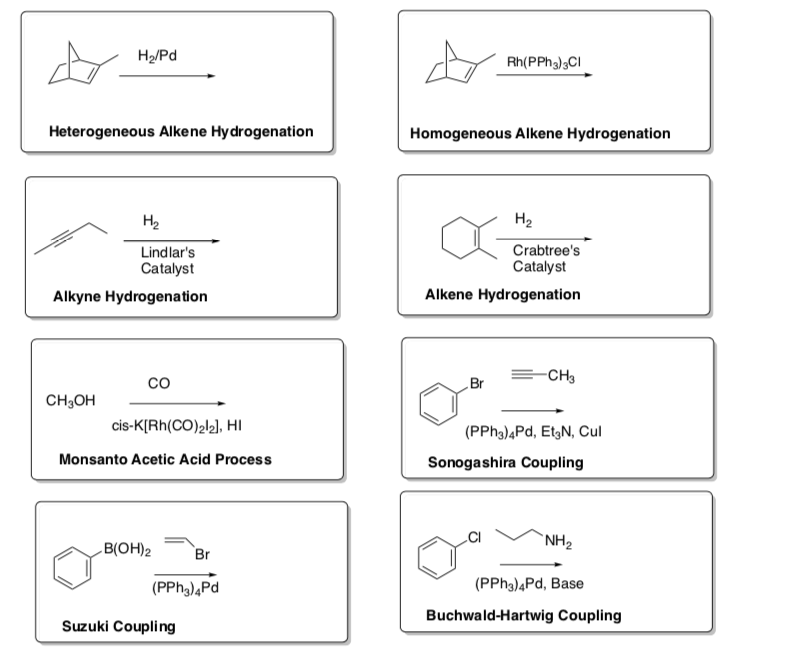
Summary of the Catalytic Cycle Notecard Reactions
- Predict the products for these reactions. Make notecards for these reactions.
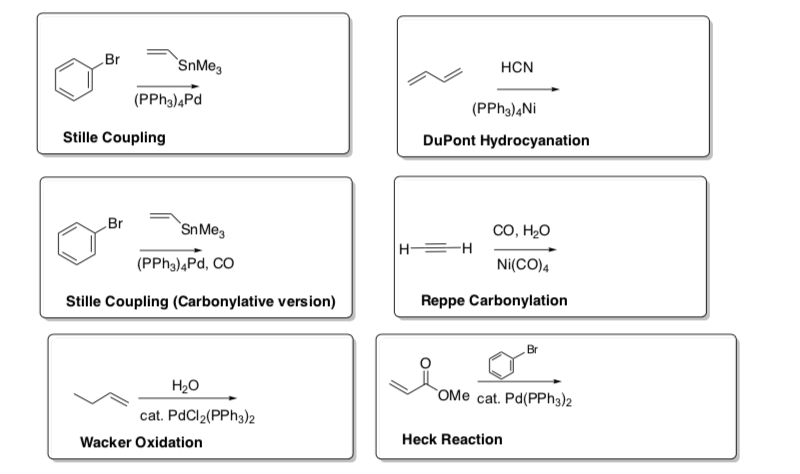
Summary of the 7 Mechanistic Steps of Catalytic Cycles
- What are the 7 mechanistic steps often used in a catalytic cycle?
- How can you tell the difference between a reductive elimination and a b- elimination?
- How do you know what order the steps occur?
Application Problems
- Hydroformylation
We did this earlier with Co but it can also be catalyzed by a Rh catalyst.
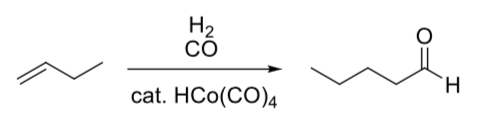
- Fill in the hydroformylation catalytic cycle.
- Label each step as: association, dissociation, reductive elimination, oxidative addition, 1,1-insertion, or 1,2-insertion.
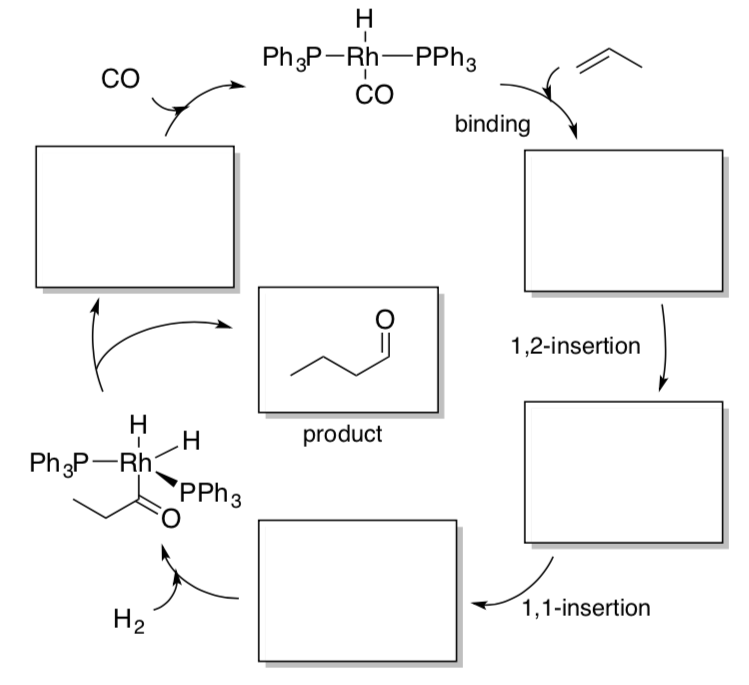
- Use of Catalytic Reagents in Synthesis
- Complete the following synthesis by filling the empty boxes with appropriate reagents.
Bisai, V.; Sarpong, R. Methoxypyridines in the Synthesis of Lycopodium Alkaloids: Total Synthesis of Lycoposerramine R Org. Lett., 2010, 12(11), 2551-2553.
- Complete the following synthesis by filling the empty boxes with appropriate reagents.
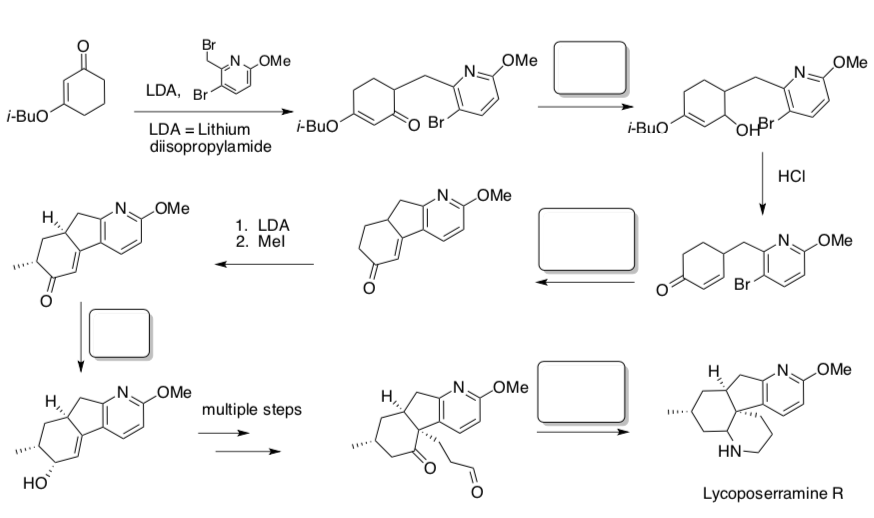
- Buchwald-Hartwig Side Reaction
A side reaction can occur wherein β-hydride elimination followed by reductive elimination produces the hydrodehalogenated arene and the corresponding imine.
- Identify the steps that differ from the traditional Buchwald-Hartwig.
- What differs about the metal complex?
- Label the β-hydride elimination followed by reductive elimination that leads to the imine side product.
- Typically β-hydride elimination requires an open coordination site for the newly forming ligand. Explain why this catalytic cycle is more likely to lead to the imine than the previously studied process.
- Another catalytic cycle
- Fill in the blanks on this reaction.
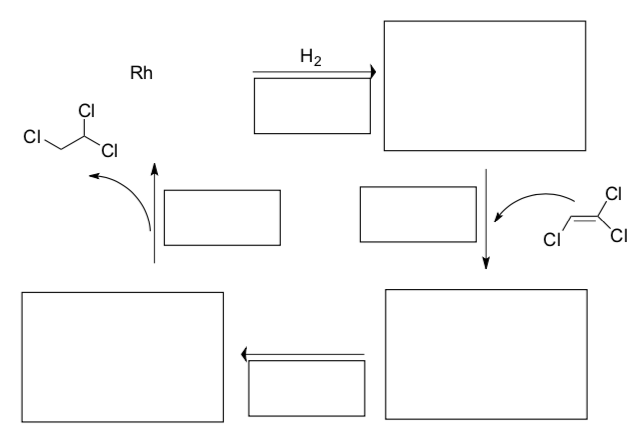
- Fill in the blanks on this reaction.
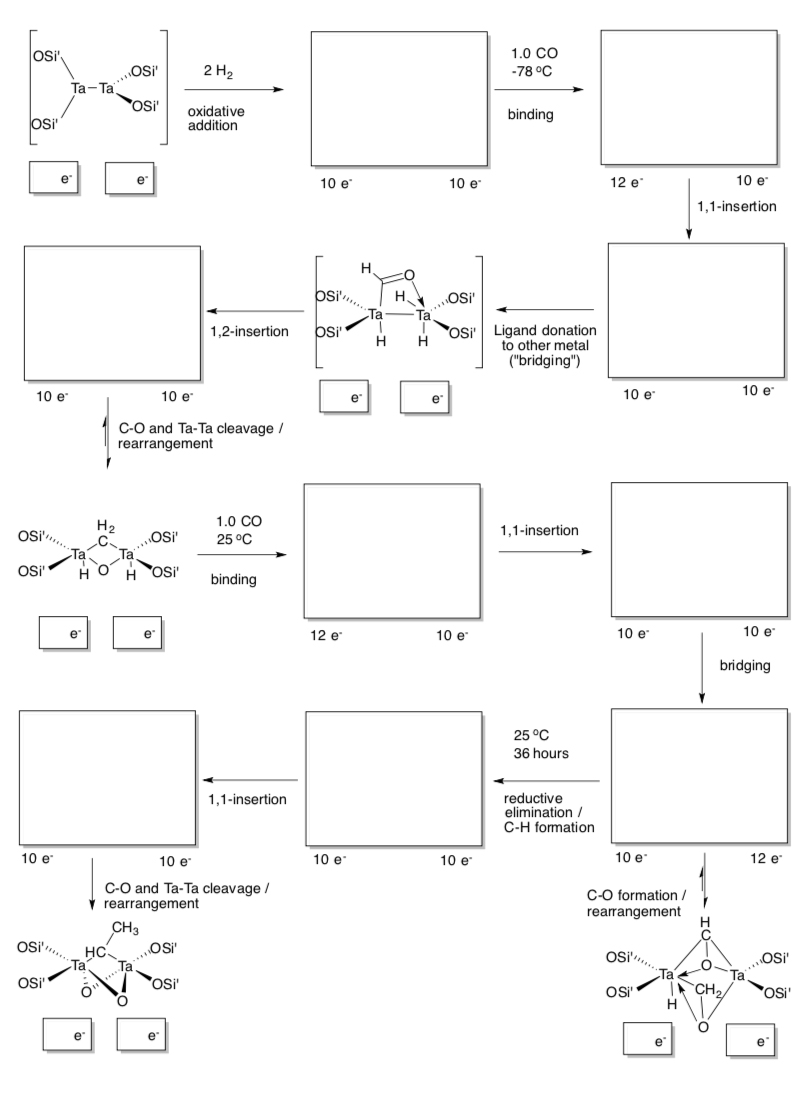
- Fischer-Tropsch
A company in Briatin recently announced plans to couple CO2-capture technologyy to The Fischer-Tropsch methods of making hydrocarbon fuels from oxygenates. This could lead to carbon neutral fuels.
- Fill in the boxes on this Fischer-Tropsch (JACS, 1987).

- Fill in the boxes on this Fischer-Tropsch (JACS, 1987).


