19: Kineticscatalyst
- Page ID
- 144242
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Kinetics of Organometallic Catalysis
- Catalysis is the change in rate of a chemical reaction due to the participation of a catalyst.
There are many reactions in chemistry that are thermodynamically favored yet occur slowly at room temperature.
For example:
Hydrogenation of an alkene

Glucose Metabolism
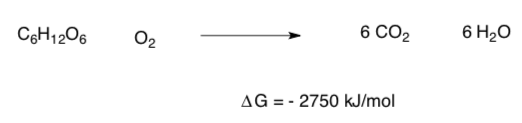
- Generalization: ΔG <_________________kJ mol-1 is spontaneous and product favored.
- Are these two reactions product favored?
- Does glucose typically spontaneously combust? Why or why not?
- Catalysts interact with substrates to provide a different reaction pathway that results in a significantly [ higher / lower ] free energy of activation (ΔG‡) than the uncatalyzed pathway.
The presence of a catalyst increases the rate of the reaction even those that may not have negative ΔG.
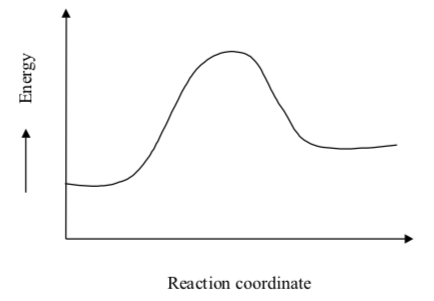
Reaction Profiles of Catalyzed Reactions

- Which is the catalyzed reaction?
Solid line Dashed line
There are three ΔG values labeled on the graph.
- Clarify what each value is and how it is different from the others.
- ΔG‡:
- ΔGc‡:
- ΔGrxn:
- Put these labels on the correct place on the graph:
- S and C (substrate and catalyst)
- S-C Complex
- P and C (product and catalyst)
- P-C Complex
Rate of Catalyzed Reactions
- As the activation barrier (G‡) is lower in a catalyzed reaction than in an uncatalyzed reaction, predict:
- How many molecules will get over the barrier?
- How quickly are the molecules likely to get over the barrier?
- How easily will the molecules be able to go over the barrier in the reverse direction?
- What happens to the rate of this reaction?
Speeds up | Stays the same | Slows down
- What happens to the rate of the reverse reaction?
Speeds up | Stays the same | Slows down
- If the rate increases in the forward and the reverse reaction, does the equilibrium shift? Explain.
- Compare the DGrxn for the catalyzed reaction to the DGrxn of the uncatalyzed reaction:
Same | Catalyzed is larger | Uncatalyzed is larger
Remember the relationship between ΔG and K (equilibrium constant):
$$
\Delta G=-R T \ln K \quad R=8.314 \frac{J}{\operatorname{mol} \cdot K} \quad T=298 K
$$
- Compare K (equilibrium constant) for the catalyzed and uncatalyzed reactions:
Same | Catalyzed is larger | Uncatalyzed is larger
- Define Keq:
Keq =
- At equilibrium, is the ratio of product to starting material different when a catalyst is present?
Reaction Curves

- Which of these curves represents the catalyzed reaction?
- Compare the final amount of substrate in each reaction (assuming you reach equilibrium).
- Compare the half-life of each reaction (the time it takes to consume half the substrate).
Rate Laws – Reversible Steps?
Here is the equation of nitric oxide reacting with oxygen to produce nitrogen dioxide.
2NO(g) +O2 -> 2NO2(g)
rate=k[NO]2[O2]
- What would be the order of this reaction?
- How likely is it that this really occurs in one step?
Instead, we can suggest a mechanism with two elementary steps that are bimolecular.

- What is the net reaction for the newly proposed reaction?
- Which is the rate determining step?
If Step 2 is the rds, then rate law would be k2[N2O2][O2], which does not seem to match with the experimental rate of k [NO2]2[O2]. However, N2O2 is actually a reaction intermediate and an intermediate cannot be in the rate law for the overall reaction.
We want to have a value that is equivalent to [N2O2] to substitute into the rate equation. Since Step 1 is a fast, reversible reaction, we can make the assumption that:
k1 [NO]2 = k-1 [N2O2],
so [N2O2] = (k1/k-1) [NO]2
- Through substitution, k2[N2O2][O2] =
- Does this match the experimental rate law?
Rules for writing rate laws for multi-step reactions with reversible steps: The rate law includes only the starting materials needed up to rds. The rate constants for forward steps (k) are multiplicative and are in the numerator; while the reverse steps are in the demoninator and are additive.
Rate Laws for Catalyzed Reactions
Catalysts participate in the reactions in a cyclic series of steps: association (binding), bond-making/bond-breaking steps and dissociation.
- Based on the generalized reaction progress diagram on a previous page, which step is likely to be the rate-determining step?
Binding | Bond-making | Bond-breaking step(s) | Dissociation
Olefin isomerization is an important process in petroleum refining. Consider the following catalytic cycle.

There are a lot of steps here. Let’s look at them one at a time.
- At least one of these steps shown, k4, might be reversible. However, k4 is probably very rapid in the direction shown. Explain why.
- At the other end, k1 probably has a pretty low barrier. Explain why.
- For simplicity, assume that k2 and k3 are irreversible. Compare k2 with k3. If the Pd-H bond strength is estimated at 65 kcal/mol and the C-H bond strength is estimated at 95 kcal/mol, which step do you think is rate determining?
- Propose a rate law for this reaction.
- Can you simplify this rate law?
When dealing with catalytic systems, we frequently simplify the kinetics to two basic terms: Kb (the catalyst-substrate binding constant) and kcat (everything else).
- Identify Kb and kcat in your rate law.
Turnover
Catalysts participate in the reactions in a cyclic series of steps: association (binding), bond-making/bond-breaking steps and dissociation. During each catalytic cycle, the catalyst is regenerated. Each cycle is called a turnover.
In organometallic catalysis, turnover number (abbreviated TON) is the number of moles of substrate that a mole of catalyst can convert before becoming inactivated. The term turnover frequency (abbreviated TOF) is used to refer to the turnover per second.
- Suppose you need to make 100 moles of benzaldehyde for your new perfume plant, and you decide to make it via cobalt-catalysed oxidation of benzyl alcohol. How many moles of cobalt catalyst will you need if it has a TON=10,000?
Saito and co-workers reported the following TOFs for the dehydrogenation of heptane to 1-heptene catalysed by Rh(L)2CO(Cl):
L = PMe3: 795 hr-1; L = PEt3: 466 hr-1; L = PPh3: 136 hr-1.
- Which catalyst is most efficient?
- Propose a reason for the differences between these three catalysts.
Homogeneous and Heterogeneous Catalysis
A heterogeneous catalyst is present as another phase in the reaction (e.g. a chunk of metal or mineral).
A homogeneous catalyst is dissolved in the reaction along with the reactants (e.g. a coordination compound or some other soluble chemical).
- In the following two reactions:
- Circle the catalyst.
- Match the following reactions to the type of catalyst: heterogeneous or homogeneous.

Characteristics of Homogeneous vs Heterogeneous Transition Metal Catalysts
Characteristic Homogeneous Heterogeneous Composition discrete molecule nondiscrete molecule Active site well-defined not well-defined Properties easily modified difficult to modify Selective unselective Thermally unstable thermally robust Mild conditions extreme conditions Ease of Separation Difficult Easy
- If you could summarize even further, why might you choose to use a heterogeneous catalyst in your new production facility?
- How would you separate the heterogeneous catalyst from the rest of the reaction?
- Why might you choose to use a homogeneous catalyst in your new production facility?
- Suppose your homogeneous catalyst is a coordination compound, like Pd(PPh3)4. Why might it be thermally unstable?
- Suggest one way in which homogeneous catalysts can be made much more selective.
- “Well-defined” means the catalyst’s structure has been studied in detail.
- Explain some techniques that may have been used to study it.
- Why wouldn’t these structural techniques work on the heterogeneous catalyst?
Summary of Catalyzed Reactions
- How does catalysis affect the following reaction parameters?
- Forward reaction rate
- Reverse reaction rate
- ΔG
- ΔG‡
- Keq
- Rules for writing rate laws for multi-step reactions with reversible steps:
- The rate law includes ______________.
- The rate constants for forward steps are [ multiplicative / additive ]
- The rate constants for forward steps are in the [ numerator / denominator ].
- The rate constants for reverse steps are [ multiplicative / additive ]
- The rate constants for reverse steps are in the [ numerator / denominator ].
- Define:
- Turnover number:
- Turnover frequency:
- Heterogeneous catalysts:
- Homogeneous catalysts:
- Which type of catalysis can be modified for specific reactions (e.g. to improve regiochemistry)?
Application problem
- Catalysts and Rate of Reaction
Consider a reaction where two chemicals ‘A’ and ‘B’ react to form ‘C’.
A(aq) +B(aq) -> C(aq) ΔH > 0 (Endothermic)
Beaker says: “The catalyst changes the mechanism of the reaction in such a way that the activation energy of the reaction decreases”
Bunsen disagrees: “No, the catalyst just decreases the activation energy of the reaction without affecting on reaction mechanism”
Beaker says: “With catalysts, more product is formed”
Bunsen disagree: “No, the catalyst does not affect the yield of ‘C’”
- Explain how a catalyst affects (1) the rate of reaction, (2) activation energy, (3) the yield of product and (4) mechanisms of the reaction? Please give reasons for your answer.
- This graph shows a pathway for uncatalysed reaction. Add a second line to indicate the reaction pathway with a catalyst. Explain your drawing.
- Writing Rate Laws
The following mechanism has been proposed for the reaction of nitric oxide with hydrogen:
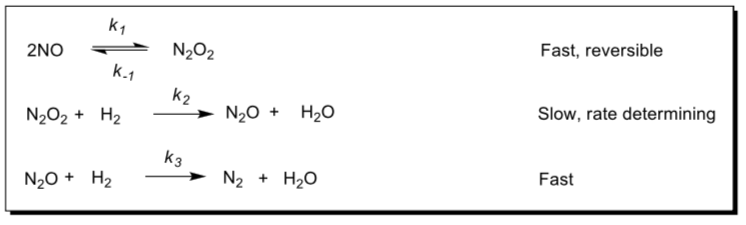
The experimental rate law for the formation of N2 is rate = d[N2]/dt = k[NO]2[H2]
- Write the balanced equation for the overall reaction.
- Identify all reaction intermediates.
- Show that the proposed mechanism is consistent with the experimental rate law.
- Relate the rate constant k to the rate constants for the elementary reactions.
- Rate Laws with a catalyst added
Some scientists did an experiment to find out the order of the reaction below:
2NO(g) -> N2(g) + O2(g) ∆H ‹ 0 (Exothermic)
They measured the concentration of nitrogen monoxide (NO) regularly over time.
The graph below shows how the concentration of NO changes as time passes.
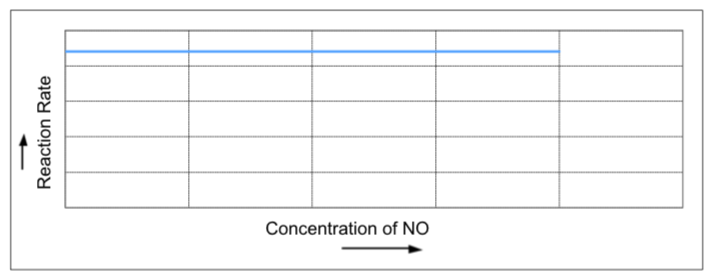
After interpreting the graph, the scientists concluded that the reaction is zero order with respect to ‘NO’ and thus the rate expression for the reaction is:
- What do you think zeroth order means?
- Write a rate law for this reaction.
- How do you think the graph supports their conclusions? Explain your answer as fully as you can.
Explain how the following conditions would affect the reaction rate. Please give reasons for your answer.
- Increasing initial concentration of nitrogen monoxide
- Increasing initial temperature of the reaction
- Increasing the amount of the solid catalyst (Pt, Platinum)
- Writing Chemical Equations
- Why doesn’t a catalyst appear in the overall chemical equation for a reaction?
- Sulfur dioxide is oxidized to sulfur trioxide in the following sequence of reactions:
2SO2 (g)+2NO2 (g) -> 2SO3 (g)+2NO(g)
2 NO (g) + O2 (g) -> 2 NO2 (g)
- Write out the chemical equation for the overall reaction.
- Identify any molecule that acts as a catalyst or intermediate in this reaction.
- What would happen to the reaction if the supply of O2 was cut off from the reaction?
- What is the role of O2 in the conversion of SO2 to SO3?


