18: Drug Design
- Page ID
- 144187
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Drug Design
Enzymes as Drug Targets
Reversible Inhibitors
Binding interactions between an enzyme and the substrate are very important. If there are no interactions holding the substrate to the active site, then the substrate will drift in and back out again before there is a reaction.
Quick Thermodynamics Refresher: (circle one in each of the statements below)
- Substrate binding through IMF is [ favored / disfavored ] due to ΔH.
- Substrate binding is [ favored / disfavored ] due to ΔS.
- With [ weaker / stronger ] binding to the transition state of the natural reaction, the likelihood of a reaction occurring [ increases / decreases ].
- Describe Transition State Theory:
A medicinal chemist aims to design a molecule (drug) which is similar to the substrate and/or transition state but which binds more tightly. It may not undergo any reaction but it blocks access to the natural substrate and the reaction cannot occur.
- Draw a cartoon of this competitive inhibition when a drug mimics the substrate but does not undergo a reaction.
Irreversible inhibitors bind irreversibly to the active site and block the enzyme permanently. The most effective irreversible inhibitors are those that can react with the side chain of an amino acid of the enzyme to form a covalent bond.
- Draw a cartoon of an irreversible inhibition when a drug binds covalently to the enzyme preventing the enzyme from further reactions.
- When might you want a drug to be an irreversible inhibitor vs a competitive inhibitor?
Enzyme Inhibitor Nuts and Bolts Activity
Adapted from Junker, J. Chem. Ed., 2010, 87(3), 294-295.
Using the nuts and bolts in the three activities below, complete the following table.
| Setup | # of Bolts Unscrewed | Time (min) | Rate or Velocity (bolts/min) | Simulated Condition |
| Activity 1 Pile of bolts | Saturated [S] | |||
| Activity 2 Glued bolts | Competitive Inhibition | |||
| Activity 3 Restrained hands | Noncompetitive Inhibition | |||
| Activity 4 Stealing | Uncompetitive inhibition |
Activity 1:
The student enzyme attempts to unscrew as many bolt-nut complexes as possible in 30 sec. The data is entered in the table.
Activity 2:
The student enzyme attempts to unscrew as many bolt-nut complexes as possible in 30 sec. If the bolt-nut complex is glued together, the student returns it to the pile and picks up a different one. The data is entered in the table.
Activity 3:
The student enzyme attempts to unscrew as many bolt-nut complexes as possible in 30 sec WHILE a second partner holds their dominant hand down.
Activity 4:
The student enzyme attempts to unscrew as many bolt-nut complexes as possible in 30 sec WHILE a second partner tries to tag the bolt-nut complex by wrapping it with blue tape. A complex can only be ‘tagged’ and removed from the pile if the student enzyme is holding it.
Competitive Inhibitors
Competitive inhibitors bind to the active site through IMF and so the binding is reversible allowing an equilibrium to occur between the bound and unbound drug. The inhibition caused by the drug is reversible.
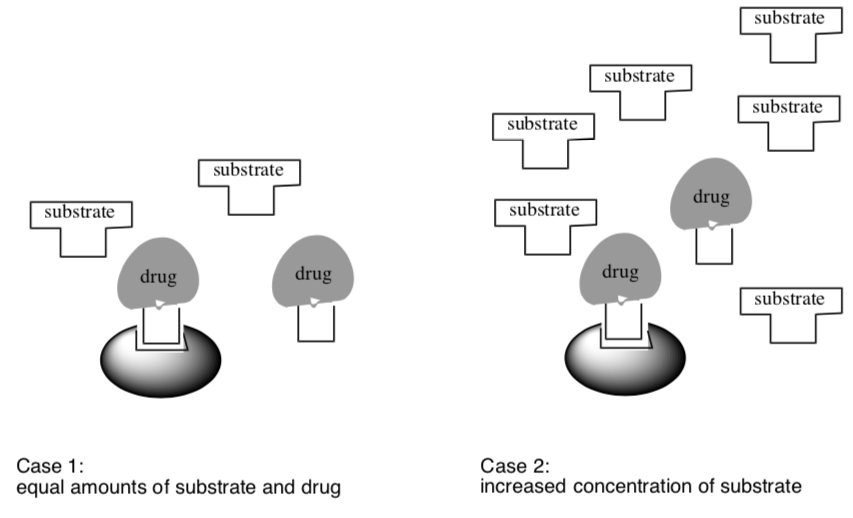
- Circle the enzyme reaction which would be faster:
Case 1 OR Case 2
- Explain why this reaction is faster.
- If you did the bolt-nut activity, make a comparison to these enzyme situations.
Graphing Rates of Competitive Inhibitors
Competitive enzyme inhibitors are used in a lot of medical applications. When designing a new drug, a chemist wants to evaluate inhibition type.
- Clearly label or highlight the curves on the following graph:
- regular enzyme reaction rate as substrate increases.
- enzyme reaction rate as substrate increases and inhibitor is present.

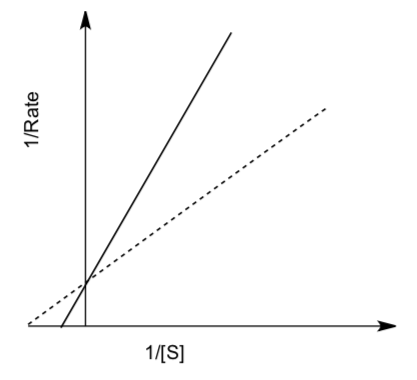
Lineweaver-Burk plots (double reciprocal plots) graph 1/rate vs. 1/[S] to give a linear graph. For competitive inhibition,
- Clearly label or highlight lines on the following graph:
- regular enzyme reaction
- enzyme reaction with competitive inhibitor present
Non-competitive Inhibition
Noncompetitive inhibition occurs when the inhibitor binds at a site distinctly different from the active site.
- This alternative site on the enzyme is generally known as ______________ site.
Binding of an inhibitor at this site generally reduces the enzyme’s ability to convert substrates into products. Noncompetitive binding occurs when the inhibitor reduces the activity of the enzyme and binds equally well to the enzyme whether or not it has already bound the substrate.

- In your own words, describe how the noncompetitive inhibition in the cartoon above prevents the enzyme reaction.
- When noncompetitive inhibitors are added to a reaction, enzyme can no longer function as well as it could in the absence of the inhibitor, so the maximum rate [ decreases / increases ].
Graphing Rates of Non-Competitive Inhibitors
- Clearly label or highlight the curves on the following graph:
- regular enzyme reaction rate as substrate increases.
- enzyme reaction rate as substrate increases and noncompetitive inhibitor is bound to the allosteric site.

- If you did the bolt-nut activity, make a comparison to this enzyme situation.
For noncompetitive inhibition,
- Clearly label or highlight lines on the following Lineweaver-Burk plots:
- regular enzyme reaction
- enzyme reaction with a noncompetitive inhibitor bound.

The y-intercept is related to the highest possible rate for the enzyme reaction.
- Explain why the two lines have a different y-intercept on the Lineweaver-Burk plot for a non-competitive inhibitor but they do not for the competitive inhibitor.
The x-intercept is related to the rate of dissociation of the enzyme-substrate complex.
- Explain why the two lines have the same x-intercept on the Lineweaver-Burk plot for a non-competitive inhibitor but they do not for the competitive inhibitor.
Uncompetitive Inhibition
Uncompetitive Inhibition occurs when an inhibitor can only bind the enzyme-substrate complex. That is, free enzyme is not a target of inhibition, but once a substrate enters so too can the inhibitor.

- In your own words, describe what is happening in the cartoon above.
- Based on the binding model, would you expect an uncompetitive inhibitor to resemble the substrate of the reaction it is inhibiting? Why or Why not?
- When uncompetitive inhibitors bind to the E-S complex, the enzyme can no longer function as well as it could in the absence of the inhibitor, so the maximum rate [ decreases / increases ].
- As the inhibitor binds to the E-S complex, the equilibrium is shifted. The ES complex is removed. Using Le’Chatelier’s Principle, what do you think will happen to the extent of completed reaction?
Graphing Rates of Uncompetitive Inhibitors
- Clearly label or highlight the curves on the following graph:
- regular enzyme reaction rate as substrate increases.
- enzyme reaction rate as substrate increases and uncompetitive inhibitor binds to the E-S Complex.

For uncompetitive inhibition,
- Clearly label or highlight lines on the following Lineweaver-Burk plots:
- regular enzyme reaction
- enzyme reaction with an uncompetitive inhibitor bound.

The y-intercept is related to the highest possible rate for the enzyme.
- Based on the method of inhibition, explain why the two lines have a different y- intercept on the Lineweaver-Burk plot.
The x-intercept is related to the rate of dissociation of the enzyme-substrate complex.
- Based on the method of inhibition, explain why the two lines have adifferent x- intercept on the Lineweaver-Burk plot.
Summary of Inhibition and Lineweaver-Burk Plots
Non-competitive inhibition differs from competitive inhibition in that the binding of the inhibitor does not prevent binding of substrate, and vice versa, it simply prevents product formation for a limited time.
- Define the different types of inhibitors:
- Uncompetitive:
- Non-competitive:
- Competitive:
Each type of inhibition produces a different Lineweaver-Burk Plot.
- Label each line as one of the following:
- Normal Enzyme
- Enzyme with competitive inhibitor
- Enzyme with noncompetitive inhibitor
- Enzyme with uncompetitive inhibitor

- Explain the significance of the changes in y-intercept.
- Explain the significance of the changes in x-intercept.
Application Problems
- Inhibitor type?

- For the cartoon reaction above of an enzyme converting its substrate into product, which type of inhibitor is acting (denoted by the star-shaped molecule)?
Competitive Noncompetitive Uncompetitive
- For the cartoon reaction above:
- What will happen to the maximum reaction rate?
- For the cartoon reaction above of an enzyme converting its substrate into product, which type of inhibitor is acting (denoted by the star-shaped molecule)?
- Mixed Inhibition
Non-competitive inhibition is a type of enzyme inhibition where the inhibitor reduces the activity of the enzyme and binds equally well to the enzyme whether or not it has already bound the substrate.
- Draw a cartoon showing non-competitive inhibition.
- Draw a Lineweaver-Burk plot showing how the non-competitive inhibition compares to the enzyme with a native substrate.
In reality, true non-competitive inhibition is very rare.
- It is unlikely that binding of an inhibitor would not also affect binding of the substrate in some way. Explain why in your own words.
In mixed inhibition, the inhibitor binds to an allosteric site causing a decrease in the affinity of the enzyme for the substrate.
- Draw a predicted Lineweaver-Burk plot showing how mixed inhibition compares to the enzyme with a native substrate.
Drug Design Case Study: Statins
Part I. Heart Disease
Heart disease is the number one leading cause of death in men and women. It is a costly health problem facing our nation today.
Part II. Enzyme Target
In 1971, Akira Endo, a Japanese chemist working for the pharmaceutical company Sankyo, began the search for a cholesterol-lowering drug. Research had already shown that cholesterol is mostly manufactured in the liver.
- Which step of a pathway is usually the regulation point?
- Based on your understanding of biochemical pathway control, which step of the cholesterol biosynthesis pathway shown below would be a good target for inhibiting cholesterol synthesis? Why?
- Circle the enzyme that regulates this pathway.

Part III. Enzyme Reaction
HMG-CoA reductase catalyzes a key step in the mevalonate pathway, which is involved in the synthesis of sterols, isoprenoids and other lipids. HMG-CoA Reductase catalyzes the following reaction:
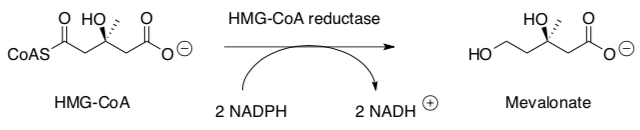
Part III. Binding of Mevalonate to the Enzyme Active Site
- Identify key IMF holding the natural substrate, HMG-CoA, into the active site.

Part IV. Discovery of a Natural Product Lead
Akira Endo hypothesized that fungi used chemicals to ward off parasitic organisms by inhibiting cholesterol synthesis in bacteria as cholesterol is used in cell membranes. Mevastatin, a metabolite isolated from Penicillium citrinum was discovered to be active.
Endo, et. al. J. Antiobiotics, 1976, XXIX (3), p 1346-1348.
A pharmacophore is an arrangement of steric and electronic features that is necessary to ensure the optimal IMF within the active site inhibit the enzyme.
- Circle the pharmacophore on these two molecules
- Draw mevastatin into the active site with key IMF indicated.

Part V. Understanding the Enzyme Mechanism
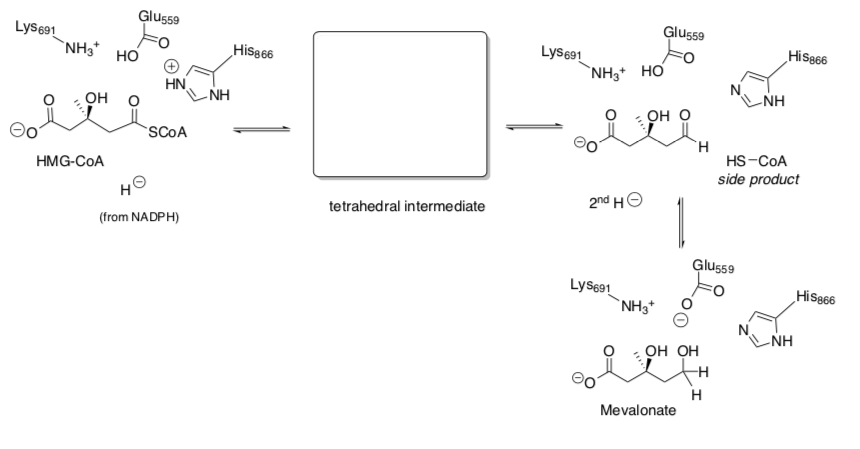
This is the reaction that is carried out by HMG-CoA Reductase
- Fill in the mechanistic arrows involved in the enzyme mechanism.
Most of the statin drugs have the following ‘polar’ head (eitherinthestructureorformedbyhydrolysisofthe lactone).
- Redraw the HMG-CoA tetrahedral intermediate from the mechanism next to the statin ‘polar head’.
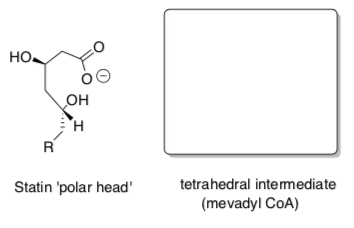 Circle the part of the molecule that resembles the transition state (or tetrahedral intermediate) of the reaction catalyzed by HMG-CoA.
Circle the part of the molecule that resembles the transition state (or tetrahedral intermediate) of the reaction catalyzed by HMG-CoA.
- Explain how mimicking the transition state of the enzyme reaction makes this a good drug (Transition State Theory).
- As these drugs bind to the active site, what type of inhibitor would they be?
Competitive /Non-competitive /Uncompetitive
- Draw a typical Lineweaver-Burk Plot for the effect of statin vs the HMG-CoA substrate on the reaction rate of the enzyme.
Part VI. Synthesis of Lipitor ®
- Atorvastatin was first synthesized by Bruce Roth (Pfizer). Sales of Atorvastatin (Lipitor) exceed $125 billion and the drug has topped the list of best-selling branded pharmaceuticals for nearly a decade.
B.D.Roth,C.J.Blankley,A.W.Chucholowski,E.Ferguson,M.L.Hoefle,D.F.Ortwine, R.S. Newton, C.S. Sekerke, D.R. Sliskovic, C.D. Stratton, M.W. Wilson, J. Med. Chem., 1991, 34, 357–366.

- Ribonuclease Inhibitor Drug Design
Part 1. Drug Target
In recent years, the members of the RNase family have attracted considerable biomedical interest as targets for the discovery of new pharmaceuticals for the treatment of inflammatory disorders and cancer. The fact that the ribonucleolytic activity of these enzymes is a prerequisite for the pathological activities related to the proteins of this family has triggered a structure-assisted approach to the design of inhibitors.
Ribonuclease catalyzes the hydrolysis of RNA.
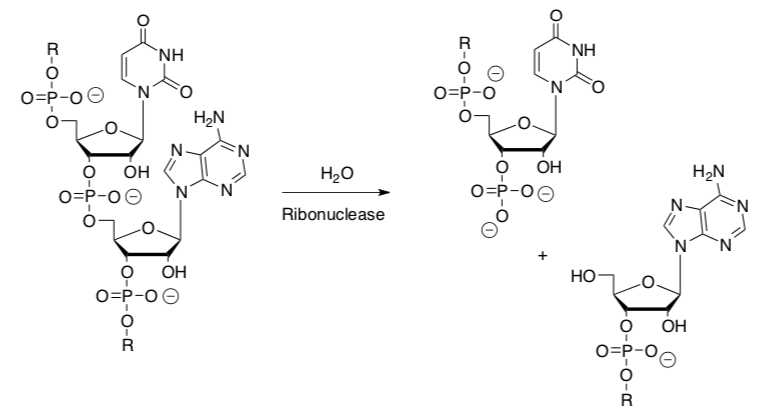
- What is a hydrolysis reaction?
- In this reaction, circle the electrophile.
- Put a box around the nucleophile.
Part 2. Enzyme Mechanism
Ribonuclease uses a two step mechanism for the hydrolysis of the RNA.
- Draw the arrows for the mechanism for the hydrolysis.

- Why does the enzyme catalyst use a two-step process rather than the direct hydrolysis?
- What is the role of the two histidines?
Part 3.Transition State Analogs

- Which case is the easiest path through the mountain range (compared to the control)?
Case I Case II Case III
- What is being stabilized in the enzyme in each of these cases?
- Case I:
- Case II:
- Case III:
Bernhard and Orgel theorized that molecules resembling the transition state or transient intermediate will bind to the enzyme more tightly than the original substrate.
- Explain why an inhibitor should mimic the transition state.
The proposed transition state for RNAase enzymes is shown below.

- What is the geometry of the central atom in this TS?
An article describes the synthesis, characterization, and kinetic studies of a new class of stable Rhenium based complexes used as ribonuclease inhibitors.
Wentworth, Wiemann and Janda, J. Am. Chem. Soc., 1996, 118, 12521-12527

- What is the geometry of the central atom in this inhibitor?
- Indicate two reasons why this complex might be a good TS analog:
From as early as 1973, complexes of uridine with oxovanadium-(IV) and vanadium(V) ions have been shown to be “potent” inhibitors of RNAases.
- What is the d electron count on these complexes based on the charge?
- Oxovanadium (IV): ____________
- Vanadium (V): ____________
- Oxorhenium (V): ____________ (this is the new drug!)
The vanadium complexes are highly labile. This new rhenium complex was much less susceptible to ligand exchange.
- Draw a splitting diagram for the oxovanadium IV, vanadium (V) and the oxorhenium (V) complexes assuming trigonal bipyramidal geometry. Splitting diagrams are in the tables at the beginning of the workbook.
- Using LFT, explain why the Rhenium V complex is more stable than the vanadium and oxovanadium compounds.
Part 4. Synthesis of the Rhenium Inhibitors
Chen, Hansske, Janda and Robins, J. Org. Chem., 1991, 56(10), 3410-3413.

Part 5. Lineweaver-Burke Plot for the Re inhibitor

- Based on the Lineweaver-Burke plot, what type of inhibitor is the Re complex?
Competitive Non-competitive Uncompetitive
- In this type of inhibition, why does the Vmax(enzyme maximum rate) remain unchanged?
- Bryostatins
The bryostatins are a class of marine natural products isolated from Bugula neritina, a bryozoan (“moss animal”). Some bryostatins display potentially useful pharmaceutical properties, being effective against some tumours and HIV.
It is believed that bryostatin 1 acts on the enzyme, Protein Kinase C 1 (PKC1). PKC1 plays a role in numerous pathways but seems to be involved in “turning on” oncogenesis (tumour formation).
- In general, what is the substrate of a protein kinase?
- What does the protein kinase do to the substrate?
- As a result, what important biochemical role is played by protein kinases?
Bryostatin 1 is a non-competitive inhibitor of PKC1. It appears to bind in the same site as diacylglycerol and phorbol acetate, activators of PKC1.
- How does this competitive binding affect PKC1 activity? Increase or decrease?
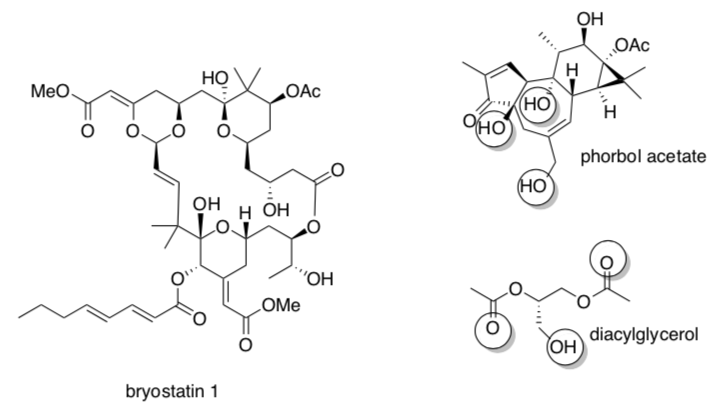
The pharmacophore of phorbol is circled.
- Draw in some amino acid residues that might bind phorbol acetate in PKC1.
- Circle the possible pharmacophore on bryostatin 1.
- Fill in the data that you would expect to see on the graphs below.


The inhibitory constant, Ki, is defined as the equilibrium constant for the following equilibrium:

in which E = enzyme; I = inhibitor.
- Write the expression for Ki.
- A larger Ki means the inhibitor is bound (more tightly / less tightly).
- A larger Ki means the inhibitor is (more effective / less effective).
The Wender Lab at Stanford began bryostatin syntheses to supplement the tiny amount available from nature. In addition to synthesizing samples of the natural bryostatins, they developed several new analogs.(Acc Chem Res 2015, 48, 752)
- Overall, these inhibitors are bound [ extremely tightly / tightly / loosely / extremely loosely ] (note that nM means 10-9 M).
- Circle the parts of each molecule that differ from bryostatin 1.
- Describe the change in Ki as minor, medium or major.

- Draw the pharmacophore – what you consider to be the essential, bare-bones part of the inhibitor (i.e. design your own bryostatin analog).
The total synthesis of bryostatin 1 took 79 to 89 steps, depending on the route. The synthesis of analog 3 took 23 steps.
- Which inhibitor would be most cost-effective?
- Complete the synthesis of analog 3. (Org. Lett. 2014, 16, 5136)

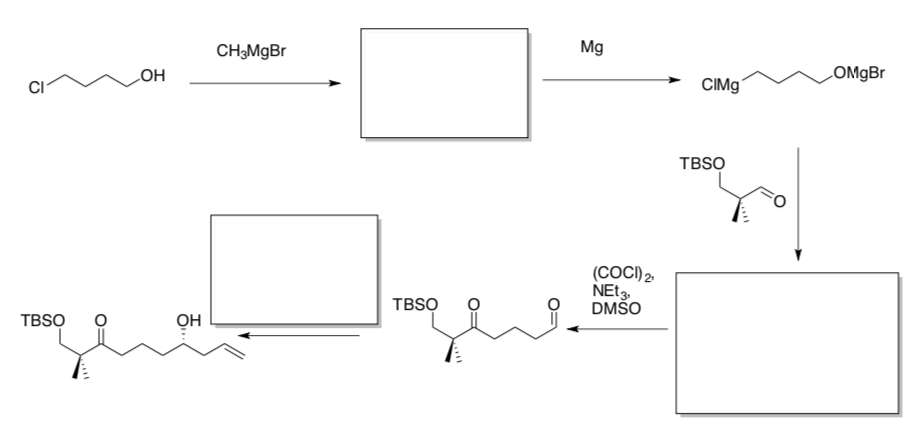
- Continue the synthesis of analog 3.


- Continue the synthesis of analog 3.



