13: Preparing LG
- Page ID
- 144070
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCWhat if there is no LG?
Three Ways to Convert an Alcohol to a LG
Hydroxyl groups are bad leaving groups. We will look at three synthetically useful ways to convert an alcohol into a good LG.
- Review: The –OH can be converted to –OTs using p-TsCl. The product of this reaction is an alkyl tosylate ester.

Common Sulfonate Ester Leaving Groups

- Draw a mechanism for the formation of a tosylate ester. Be sure to include Et3N in the mechanism.

- Why didn’t the stereochemistry change?
- Draw the structure of the methylsulfonate ester of 3-methyl 2-butanol and the trifluoromethylsulfonate ester of 3-pentanol.

- Draw a mechanism for the formation of a tosylate ester. Be sure to include Et3N in the mechanism.
- The –OH can be converted to –X (X=halide) using PCl3 or PBr3 or SOCl2. A mechanism for the reaction of bromobutane with PBr3 is shown below.
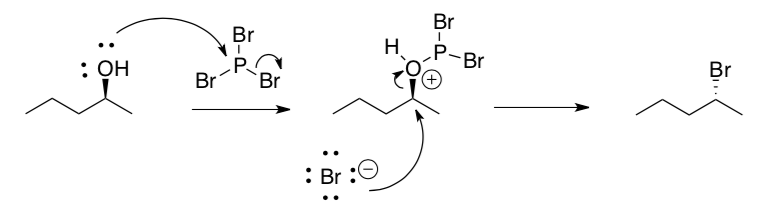
- What happened to the stereochemical center? Why?
- Draw a mechanism for the reaction of 1-butanol with PCl3.
- Draw a mechanism for the reaction of 1-propanol with SOCl2.
- Mitsunobu
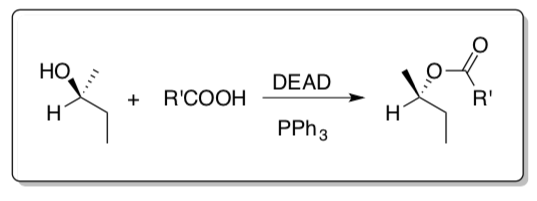
The Mitsunobu reaction allows the conversion of alcohols into esters or azides with clear inversion of the stereochemistry.
- Draw the mechanism for the Mitsunobu reaction below.
- Clearly identify when the oxygen has become a good leaving group.

This is a common side-product of the Mitsunobu reaction.

Summary of Reactions to Convert an Alcohol to a Good LG
- Compare the stereochemistry changes:
- Formation of tosylates:
- Bromination/Chlorination:
- Mitsunobu:
- Propose a reasonable explanation for the differences in stereochemical results.
- What is the biological method for converting an alcohol to a good LG? What are the stereochemical outcomes for this process?
- Predict the products for these reactions. Make notecards.

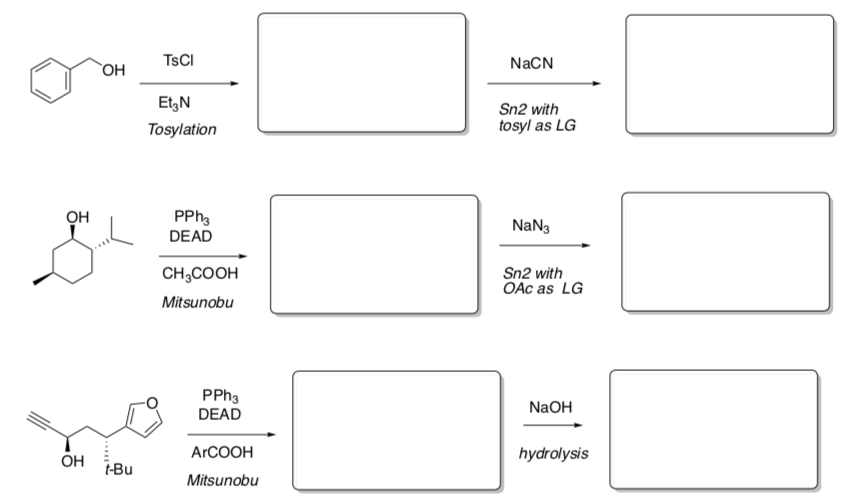
Practice Two Step Reactions

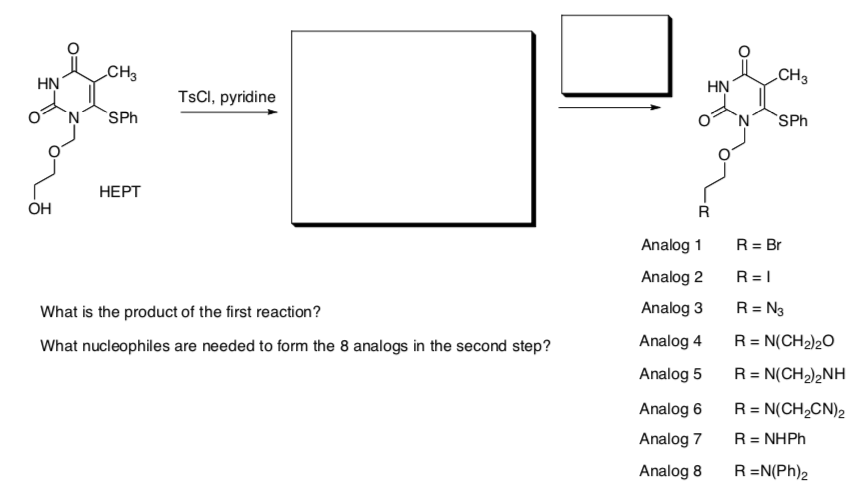
Substitution Reactions in Synthesis
Inhibition of HIV reverse transcriptase has proven to be an effective way to block viral multiplication. New anti-HIV agents are needed for resistant strains of the virus. Analogs of HEPT provide a possible source of new anti-HIV drugs.
Pontikis, et. al., Journal of Medicinal Chemistry, 1997, 40, 1845-1854.
Roadmap Practice: Apliminal
- Fill in the blanks for the following roadmap for the synthesis of (-)-apliminal.

- Answer the following questions about the reaction in the box (above).
- Draw a Lewis Dot structure for CH2N2.
- Propose a mechanism (Hint: this transformation involves a nacid-base reaction and a substitution).
Biological Leaving Groups: Phosphorylation
Proteins can be covalently modified in the cell by the addition of hydrocarbon chains in order to enhance their interaction with cell membranes (as seen in Reactivity 1). One of the most well-known examples is the prenylation of Ras proteins These proteins can function as on/off switches for cell proliferation, and so studies of this process have significance in cancer research.
In Ras proteins, a cysteine residue is farnesylated as shown:

- Circle the leaving group. Why is this a good leaving group?
- Put a box around the nucleophile.
- Provide reaction arrows and any missing intermediates for this covalent modification.
However, the farnesol (or other hydrocarbon chains) are usual found as alcohols. The cell must convert this alcohol into a good leaving group as shown below.

- Provide reaction arrows and any missing intermediates to fill in this phosphorylation. (Note: The process shown here is simplified. In reality, a smaller alcohol is phosphorylated first and then built onto using reactivity you don't know about yet to become the larger farnesyl pyrophosphate.)
- Explain with drawings (including a lipid bilayer cell membrane) why farnesylation enhances the interaction of protein with a cell membrane.



