14: Nu Sub Synthesis
- Page ID
- 144083
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
SN2 Reactions in Synthesis
Oxygen as a Nucleophile
Formations of Alcohols from Alkyl Halides

- Direct substitution
- Write a general mechanism for the formation of an alcohol from an alkyl halide with hydroxide as the nucleophile.

Hydroxide and alkoxide ions works well for this reaction with primary bromides and some secondary bromides. However, tertiary bromides tend to lead to elimination.
- Draw the elimination product for this reaction.

- Explain why this reaction favors elimination over substitution.
- Write a general mechanism for the formation of an alcohol from an alkyl halide with hydroxide as the nucleophile.
- Another classic method for converting alkyl halides to alcohols is through acylation and hydrolysis.
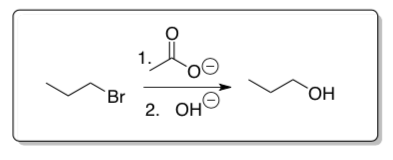
- Draw a mechanism for this reaction. Be sure to show the intermediate.

- Explain why this approach might be preferable to hydroxide ion in one step, particularly for secondary substrates.
- Draw a mechanism for this reaction. Be sure to show the intermediate.
Formation of Ethers
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol – an Sn2 reaction.

To use an alkoxide as a nucleophile, an alcohol must first be deprotonated.
- Draw the acid-base reaction needed to prepare an alkoxide ion.

NaOH is not a strong enough base to fully deprotonate an alcohol. Alcohols typically have a pKa of 16-17; the pKa of H20 is also about 16.
A stronger base is needed.
- Use a pKa table to list at least two bases that would be strong enough to form sodium ethoxide from ethanol.
- Write a general mechanism for the formation of an ether from an alkyl halide with an alkoxide as the nucleophile.

- Propose an effective synthesis of tert-butyl methyl ether,

Practice Problems
- Predict the product for each of the following ether formation reactions.
Note: The order of deprotonation vs nucleophilic substitution is important.
Nitrogen as a Nucleophile
Alkylation of Amines
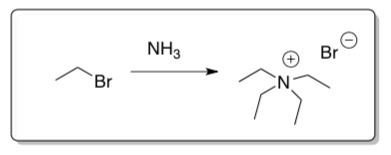
Ammonia and unhindered amines are good nucleophiles.
- Draw the curved arrow mechanism of ammonia reacting with ethyl bromide.
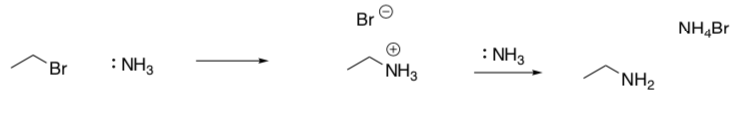
The problem with this reaction is that one can get multiple alkylation.
- Complete the following reaction pathways if you have an excess of ethyl bromide. Include intermediate products and curved arrows.

Gabriel Synthesis

Because of the problem of multiple alkylation, the Gabriel synthesis is usually used for synthesis of primary amines.
- Draw the mechanism for the reaction of butyl bromide with phthalimide and the subsequent hydrolysis in the second step.
Step 1:

Step 2:

This reaction always results in single alkylation yielding a primary amine as the product.
- The product of the first reaction, the imide, is a poor nucleophile and will not react with a second butyl bromide. Explain why.

- The product of the first reaction, the imide, is a poor nucleophile and will not react with a second butyl bromide. Explain why.
sp Carbon Nu:

Reactions involving Carbon Nucleophiles and Carbon Electrophiles are important because these reactions form new C-C bonds thus a complex carbon skeleton can be assembled.
- Draw mechanisms for the four reactions of sp Carbon Nucleophiles shown above. Don’t forget the acidic workup.
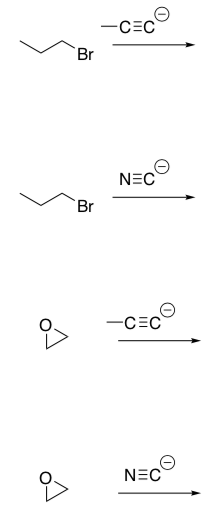
- Provide a synthetic route for converting ethyne into 3-nonyne.

Organometallic Carbon Nu:

Organometallic reagents react with epoxides.
- Provide a mechanism for these two reactions of organometallic reagents with oxirane (shown in boxes at the top of the Opage).

- These are SN2 reactions. Would you expect nucleophilic attack at site a or site b in the reaction below? Explain your choice.

As organometallic reagents are very strong bases, they usually do not do SN2 reactions with alkyl halides.
- Predict the product for the reaction of butyl lithium with 2-bromobutane.

Summary: Synthetically Useful Sn2 Reactions
- Your turn. Predict the products of these new SN2 Reactions. Then turn them into notecards.


Application Problems
- trans-Compound 2 is transformed into epoxide 3 when treated with NaH.

Let’s look at the first step of this reaction more carefully. We first added sodium hydride to the alcohol to deprotonate the alcohol. This process is highly exothermic.
- Draw the two chair forms of 2.Draw a mechanism using arrow formalism for the reaction of 2 to form 3. Be sure to clearly show which chair is involved. You might want to build these structures. You might want to build these structures.
- The corresponding cis compound 4 fails to give the corresponding epoxide. using chairs.

- The trans compound 5 also fails to undergo expoxide formation. Explain using chairs.

- Sketch an energy vs. reaction progress diagram for the two steps needed to convert 2 into the epoxide 3.
- Sodium hydride is called an ‘irreversible base’. That is to say, when it does deprotonate something, it does so irreversibly. Use LeChatelier’s Principle to explain this looking at the equilibrium between starting materials and products.
- How would using KOH for the deprotonation instead of sodium hydride affect the equilibrium?
- Formation of amines:
- When cyclopentyl bromide is treated with ammonia, a product (C5H12NBr) is formed. Treatment with base liberates cyclopentylamine which can be extracted into an organic solvent. Write a mechanism for these reactions.

- Explain why cis-1,2-dibromocyclopentane and the isomeric trans- take such different paths. Your explanation should, of course, include structures of the products.

- When cyclopentyl bromide is treated with ammonia, a product (C5H12NBr) is formed. Treatment with base liberates cyclopentylamine which can be extracted into an organic solvent. Write a mechanism for these reactions.
- Leukotrienes: Nucleophilic Substitution at Epoxides
- Provide the structure of the product below. Explain stereochemical choices.

Leukotrienes are important signaling molecules involved in the inflammatory response. Inhibition of leukotrienes is an important method of preventing asthma, as illustrated by drugs such as Monteleukast (Singulaire). There is a large family of leukotrienes, and one member is derived from another biologically, as in the example below.

- Propose a structure for glutathione.
- Draw all the steps in the mechanism converting LTA4 to LTC4. Assume there is an enzyme with a nearby carboxylic acid to provide protons and a nearby amine to accept protons. You may also use water as a proton shuttle from one site to another. You can abbreviate the structure of glutathione for the mechanism.
Leukotrienes are important signaling molecules involved in the inflammatory response. Inhibition of leukotrienes is an important method of preventing asthma, as illustrated by drugs such as Monteleukast (Singulaire). There is a large family of leukotrienes, and one member is derived from another biologically, as in the example below.
- Indicate what has changed in each of these transformations.
- Provide a mechanism using tools typically found in the cellular environment (amino acids, water).
- Provide the structure of the product below. Explain stereochemical choices.
- Roadmap: 195A
trans- 195A was first isolated as a trace compound from dendrobatid frogs. Some of these alkaloids from amphibian skin have proven to be noncompetitive blockers of nicotinic receptor channels.
Holub, Neidhofer and Biechert, Total Synthesis of (+)-trans-195A, Org. Lett., 2005, 7, 1227-1229.

- Synthesis of (-)-actisonitrile
(-)-actisonitrile, a unique ether lipid, was isolated from a single specimen of the nudibranch, Actinocyclus papillatus, and shows moderate cytotoxic activity against tumor cells.
Manzo, et.al., Org. Lett., 2011, 13(8), 1897-1899.



