12: Intro to Substitutions and Eliminations
- Page ID
- 144046
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOC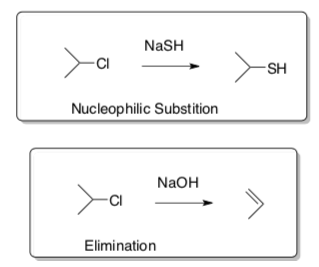

Nucleophilic Substitutions and Eliminations
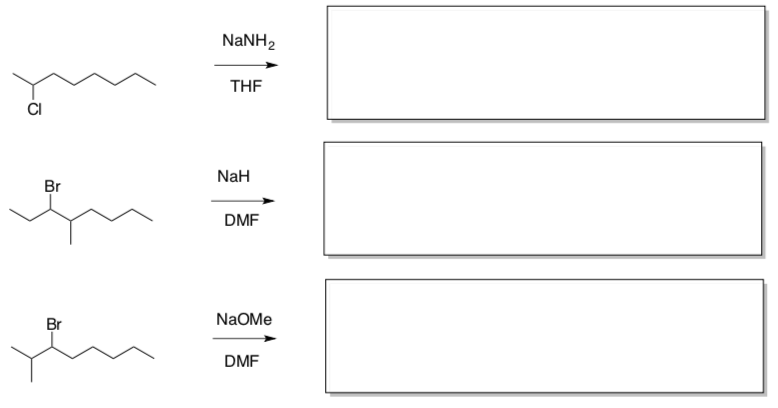
There are four competing reactions.
- Draw a mechanism using curved arrow notation for each reaction shown below.
- For each reaction, determine:
Lewis Base/Nucleophile
Electrophile
Leaving Group
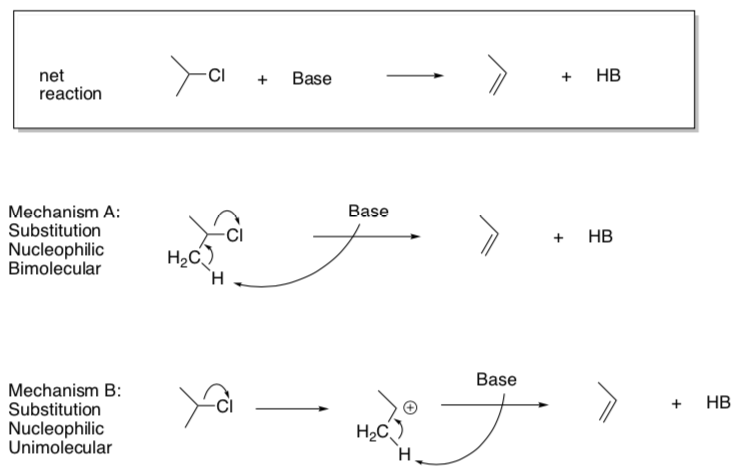
Nucleophilic Substitution
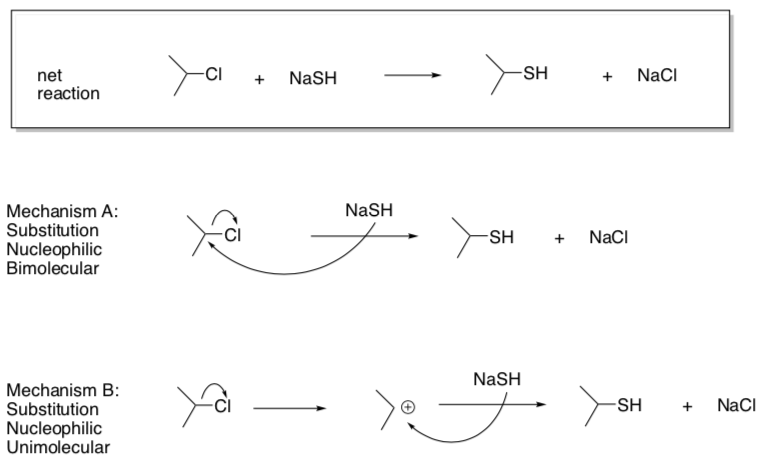
- Why can’t the nucleophile add before the bond breaking step (like Association)?
- Suggest rate laws for these two options.
Mech A: rate = Mech B: rate =
- Draw a "potential energy diagram" for each option.
- Assume the y-axis is enthalpy.
- There is always an energy barrier for each step of the reaction.
- Use your judgment to decide on relative heights of these barriers.
- Circle which step in each option will be the rds.
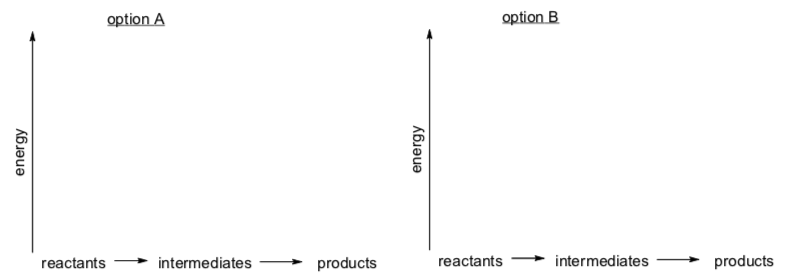
- Match these graphs with the correct substitution mechanism.
SN1 OR SN2
Elimination Reactions
- Suggest rate laws for these two options.
Mech A: rate = Mech B: rate =
- Draw a "potential energy diagram" for each option.
- Assume the y-axis is enthalpy.
- There is always an energy barrier for each step of the reaction.
- Use your judgment to decide on relative heights of these barriers.
- Circle which step in each option will be the rds.
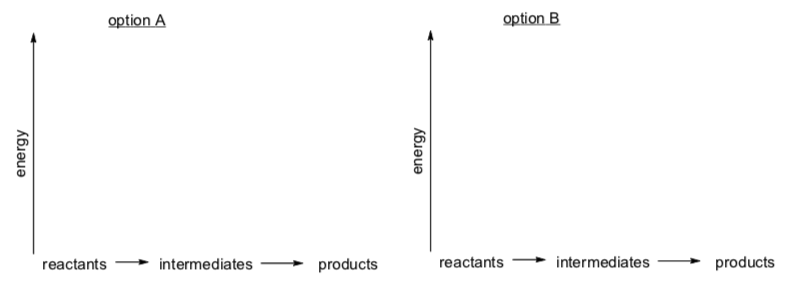
- Match these graphs with the correct elimination mechanism.
E1, the unimolecular l reaction OR E2, the bimolecular reaction
E1 OR E2
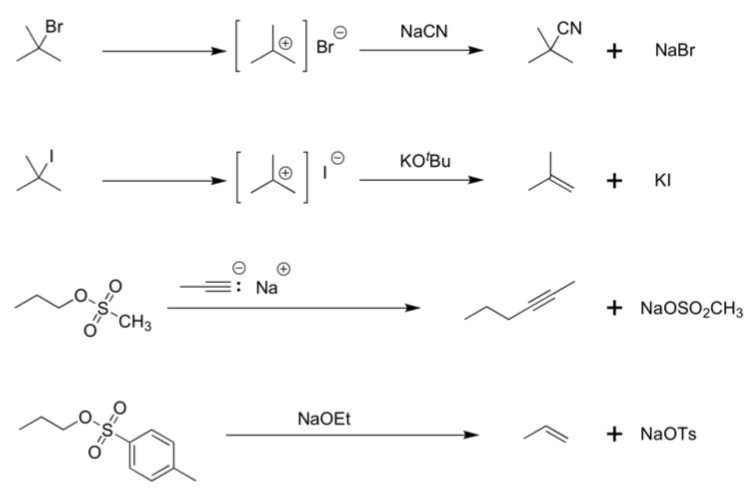
Practice
The following pair of mechanisms have been proposed for the reaction of methyl bromide (CH3Br) and hydroxide ion:
Mechanism 1: CH3Br -> CH3+ + Br- SLOW
CH3+ + OH- -> CH3OH FAST
Mechanism 2: CH3Br + OH- -> CH3OH + Br-
- What is the overall reaction?
- What is the rate law for mechanism 1?
- What it the rate law for mechanism 2?
- Which mechanism is supported by the data given below? Justify your answer. (hint: determine the experimental rate law using initial rates method)
[CH3Br]o as M [OH-]o as M Initial Rate as M/min 0.20 0.10 6.20 x 10-3 0.40 0.10 1.23 x 10-2 0.20 0.05 3.08 x 10-3
Molecular Orbital Implications of Substitutions/Eliminations: Different Stereochemical Outcomes
HOMO/LUMO calculations in understanding E+/Nu: reactions
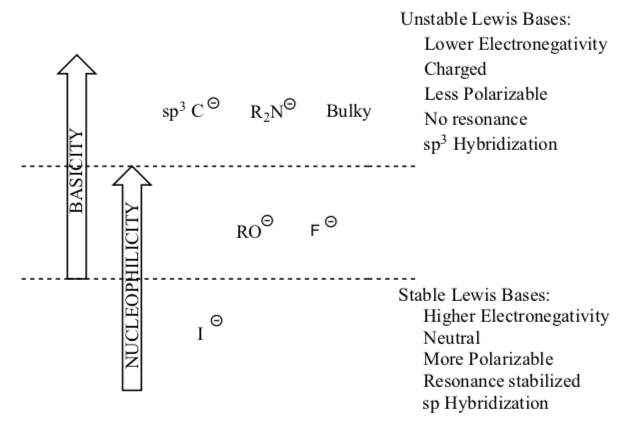
The phrase, “Lewis bases (nucleophiles) react with Lewis acids” can also be expressed: “The interaction of filled and empty orbitals is stabilizing”. This is not a trivial point – most of organic chemistry has been summarized in these few words. A nucleophile contains, by definition, a reactive pair of electrons (HOMO). Those electrons must interact with a Lewis acid, an empty orbital (LUMO).
The closer two orbitals are in energy, the more they interact and the greater the resulting stabilization. The further apart they are in energy, the smaller the interaction and the smaller the resulting stabilization. So, in general, for a good nucleophile, you want a high energy HOMO, and for a good electrophile, you want a low-lying LUMO. Under those circumstances orbital energy matching is likely to be good and the reaction fast.
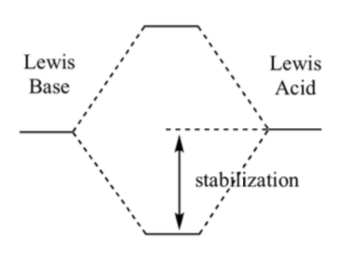
Adapted from: Maitland Jones, Jr., “Organic Chemistry”, 3rd Ed. Norton Publishers, 2005.
Molecular Orbital Implications of Substitutions/Eliminations:
An understanding of the MO of these reactions helps us understand stereochemical and regiochemical results.
NB: Pictures explaining MO of these reactions are shown in OWL and on Canvas Summary. Use these resources to take notes here.
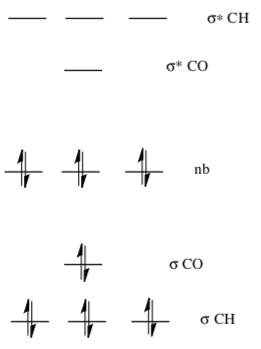
Molecular Orbitals of SN2 Reactions
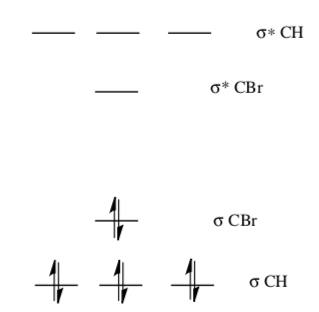
The MO energy diagram of methyl bromide is shown below.
- Circle or Highlight the LUMO (electrophilic site).
- Draw a cartoon of the LUMO.
- The MO energy diagram of methoxide is shown below.
- Identify the HOMO (nucleophilic site).
- Draw a cartoon of the HOMO.
SN2 (continued)
Explore the implications of MOs in SN2 in this reaction.
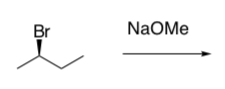
Overlap of the two orbitals creates two new orbitals.
- Show the molecular orbital diagram created by the overlap of these two orbitals.
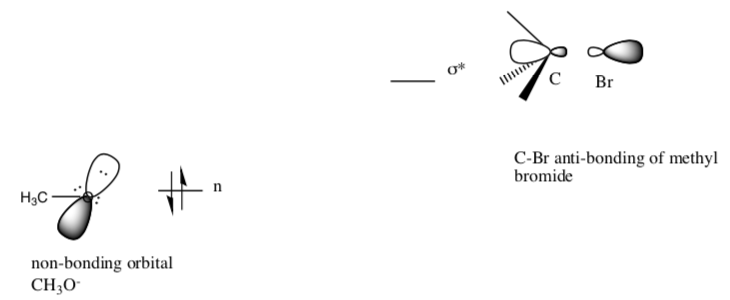
The orbitals non-bonding orbital of CH3O- could overlap in two ways with the σ * of the C-Br bond.
- Which orbital approach provides the best overlap?
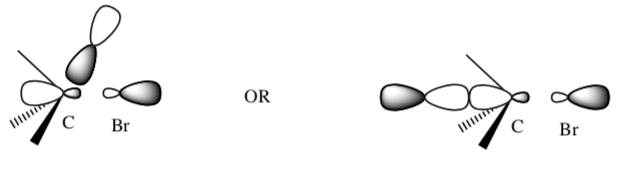
- What does MO picture tell you about the stereochemistry changes in this reaction?
- Draw the product of this reaction with the correct stereochemistry.
SN1 MO Implications
- Draw the mechanism for the SN1 reaction between 2(R)-bromobutane and 0.1 M sodium methoxide in methanol.
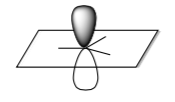
In an SN1 reaction, there is a cationic intermediate that is the electrophile (LUMO).
- Below is cartoon of the LUMO of a cationic intermediate. What is the geometry?
- Draw the cartoon of the overlap of the HOMO (non-bonding oxygen) of the methoxide with the LUMO cation on the picture above.
- Is there a preference for one of the lobes of empty p orbital?
- Draw the two possible products for this reaction.
E1 MO Implications
The E1 reaction also goes through a cationic intermediate which is the electrophile.
- Draw the mechanism for the E1 reaction between 2-bromobutane and 1.0 M sodium methoxide in methanol.
The carbocation intermediate can be drawn as a Newman Projection.
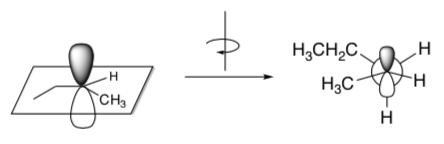
- This cation can rotate. Draw the other conformer if the back carbon rotates so that a different hydrogen atom is aligned with the empty orbital.
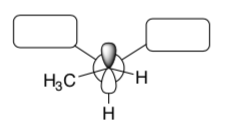
For an elimination to occur, the H atom on the carbon next door MUST be aligned with the empty orbitals so that a p bond can form.
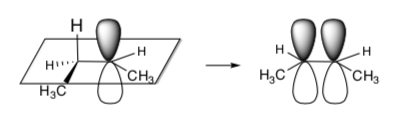
- Draw the two alkene products from both of the Newman projections above.
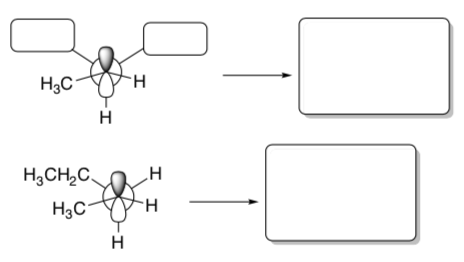
- Which one will be favored? Why?
E2 MO Implications
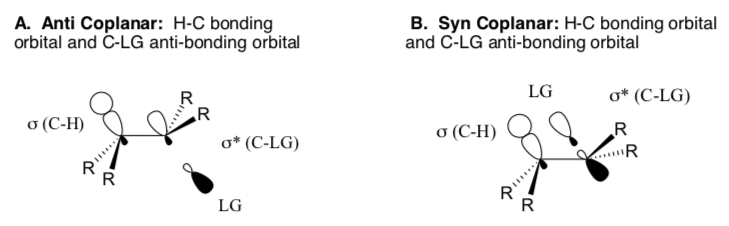
For an E2 elimination to occur, the C-H bond MUST be aligned with the C-LG bond so that a π bond can form. This is called co-planar.
There are two possible co-planar arrangements: anti and syn.
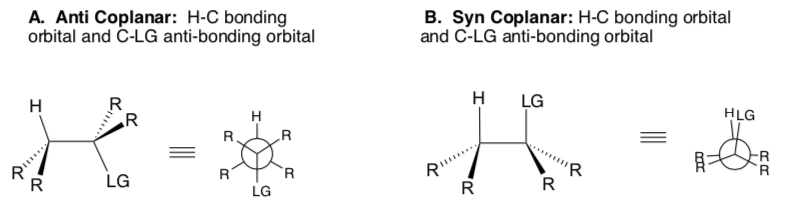
- In the picture above, circle/highlight the two sigma bonds (orbitals) that will become the π bond.
- Briefly explain why E2 reactions require that the H, C1, C2 and LG must all be in one plane (co-planar). Hint: The E2 reaction is concerted – all bond-making and bond-breaking steps occur at once.
Most E2 reactions require anti-coplanar orientation.
- Anti preference: Develop an orbital argument for why the E2 elimination favors the 180o arrangement (anti) over the 0o arrangement (syn).
E2 (cont)
Explore the implications of MOs of E2 in a reaction.
- Using curved arrow notation, draw the E2 mechanism of 1R, 2R -1-bromo-1,2- diphenylpropane with sodium methoxide.
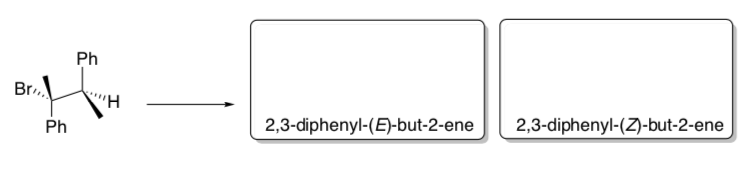
- Draw this starting material in a Newman projection as it is shown in the picture above.
- Rotate the back atom and re-draw the Newman projection so that the C-LG bond and the CH bond are anti-coplanar.
- Predict the stereochemistry of the product of this reaction.
- Predict the stereochemistry of the product of an E2 elimination when you start with this stereoisomer:
Summary of MO of Substitution and Elimination Reactions
- SN1
- Molecularity: __________
- Draw a picture of the intermediate
- Explain how this impacts the stereochemistry of the product
- E1
- Molecularity: __________
- Draw a picture of the intermediate
- Explain how this impacts the stereochemistry of the product
- SN2
- Molecularity: __________
- Draw a picture of the transition state.
- Explain how this impacts the stereochemistry of the product
- E2
- Molecularity: __________
- Draw a picture of the transition state.
- Explain how this impacts the stereochemistry of the product
Competition between Sn1, Sn2, E1, E2
Factors that Affect Substitution/Elimination Reaction Rates
1. Reactants–Hybridizationand Leaving Group
In order for any Substitution or Elimination reaction to occur, there must first be two structural requirements:
- An sp3 hybridized carbon
- A Leaving Group (LG)
A Leaving Group must be willing to take a pair of electrons when it leaves.
When different "leaving groups" are used in these reactions, the following relative rates are observed.
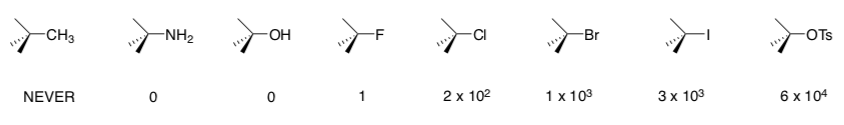
- How can you explain the different behavior induced by using different leaving groups? You may see several different trends.
- Take-Home Message: What are best categories of Leaving Groups?
- Take-Home Message: What moieties are NEVER Leaving Groups?
Common Sulfonate Ester Leaving Groups
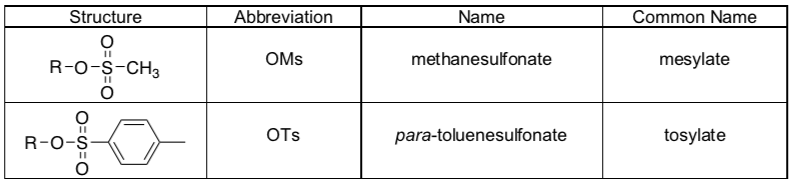
2. Reactants–Degree of Substitution
For SN1 or E1, the reactions proceed more quickly if the carbon with leaving group is
more substituted (has more carbons attached to it). Rates for a variety of reactants are shown below:
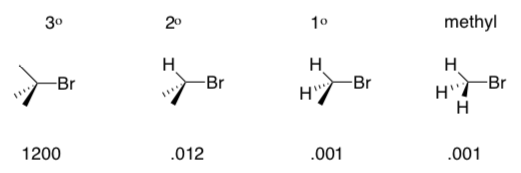
- _______ and _________ are effective substrates for unimolecular reactions.
- _______ and _________ are NEVER substrates for unimolecular reactions.
- Suggest a reason (consider the carbocation).
- Exception: Explain why the following primary substrates still undergo unimolecular reactions.

SN2 reactions proceed more quickly if the carbon with leaving group is lesssubstituted. Rates for a variety of reactants are shown below:
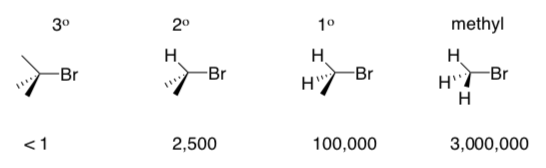
- _______ and _________ are effective substrates for bimolecular reactions.
- _________ is a mediocre substrate for bimolecular reactions.
- _________ is NEVER substrates for bimolecular reactions.
- Suggest a reason (consider the transition state).
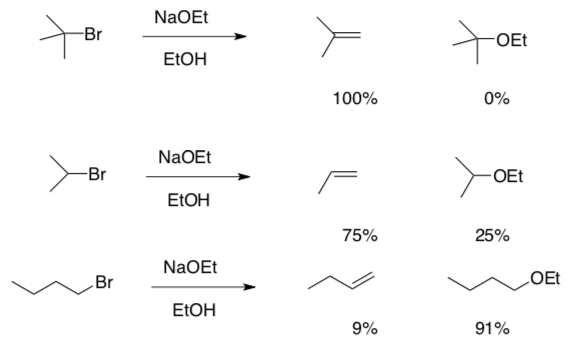
3. Steric Crowding in Substitution vs Elimination
-
Looking over the following data, comment on the role of the substrate in determining whether a compound undergoes substitution or elimination.
4. Nucleophilevs.Base
- All Bases and Nucleophiles all have a ____________.
- Eliminations require the use of a (base or nucleophile).
- Substitutions require the use of a (base or nucleophile).
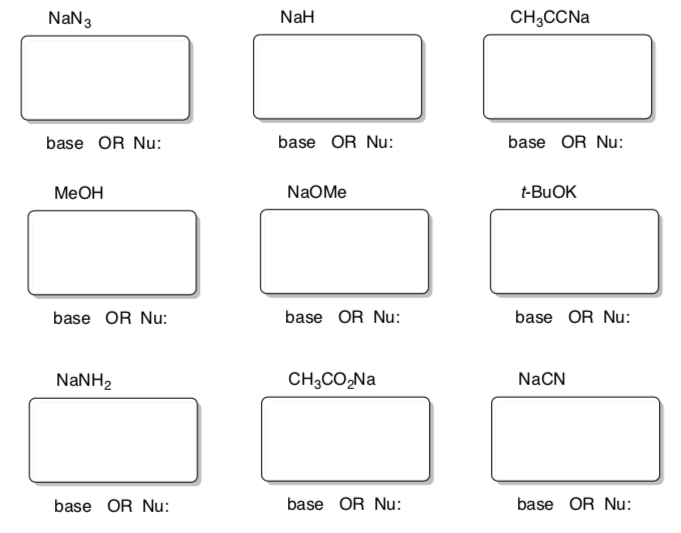
Outline for distinguishing bases versus nucleophiles:
- Draw Lewis structures for the following compounds.
- Categorize the these Lewis bases as either Nu: or B or either:
5. Solvent
For SN1 or E1, the reactions proceed more quickly if the solvent is protic (containing an OH or NH bond).
- Unimolecular reactions proceed through a _____________ intermediate. Protic solvents ( stabilize or destabilize ) the intermediate.
- Draw a picture of this intermediate solvated by water.
- Identify IMF that might help stabilize the intermediate.
Bimolecular reactions proceed more quickly if the solvent is aprotic (NOT containing an OH or NH bond).
- Bimolecular reactions are ______ step reactions (aka concerted).
- Bimolecular reactions are driven by the strength of the nucleophile or base which must contain ____________.
- Protic solvents would ( stabilize or destabilize ) the base or nucleophile which would slow the reaction down.
- However, the polar base/nucleophile must be dissolved in the solvent for the reaction to proceed. The optimal solvent is ___________ and aprotic.
Common Polar Aprotic Organic Solvents (with abbreviations)
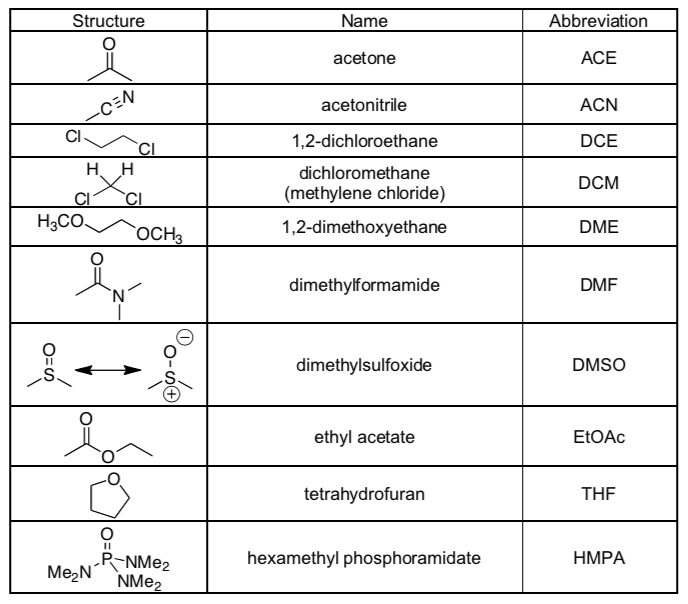
Be able to recognize these as polar aprotic solvents!
6. Temperature
Temperature favors eliminations over substitutions.
- How many bond-breaking and bond-making events occur in one step for an E2? How about SN2?
- How many products for an E2? An SN2?
- Make a thermodynamic argument to explain why temperature favors elimination.
- What is the effect of temperature on the fate of the reaction? Why?
T (oC) % substitution % elimination 45 47 53 75 42 58 100 36 64
7. Regiochemistry in Elimination
Regiochemistry refers to where in the molecule a reaction takes place.
From the following alkyl halide, two different eliminations could occur.
- Circle two protons that could be removed, leading to two different elimination products.
- Draw the two elimination products.
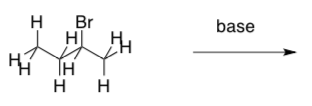
When faced with two different products, it can be useful to evaluate their relative stability. In alkenes, the order of stability is given below:
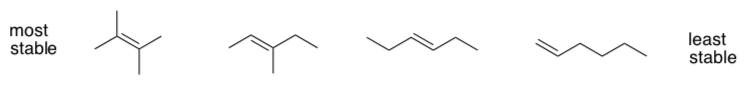
- Propose a structural factor that seems to correspond with this trend.
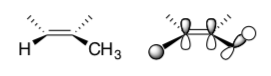
Formally, this trend is sometimes explained via hyperconjugation.
- A pi bond is a (filled / empty) orbital. It could form stabilizing overlap with a (filled / empty) orbital.
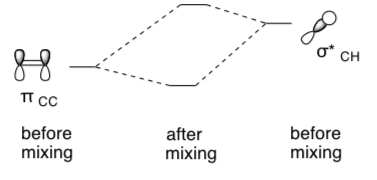
- In the following diagram, show how a conjugation effect can stabilize the alkene.
- Fill in the MO interaction diagram for this interaction with electrons.
In the following cases, more than one elimination product is possible.
- Draw the possible products.
- If one product is more stable, circle it.
In most cases, formation of the most stable alkene is favoured. Sometimes, the least stable alkene forms instead.
- Draw the possible products.
- If one product is obtained via removal of a more accessible proton, circle it.
- Overall, the _______________ stable alkene is obtained unless it is too _________________.
Rearrangements (aka 1,2 shifts)
1,2 Hydride Shifts can occur when a carbocation has formed. The H- slides from one atom to the adjacent atom (shown below).
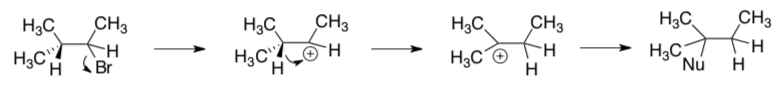
- Circle the type(s) of reactions which would have rearrangements occur.
SN1 SN2 E1 E2
- Propose a reason (driving force) for this rearrangement to occur. (Hint: consider stability of the intermediates).
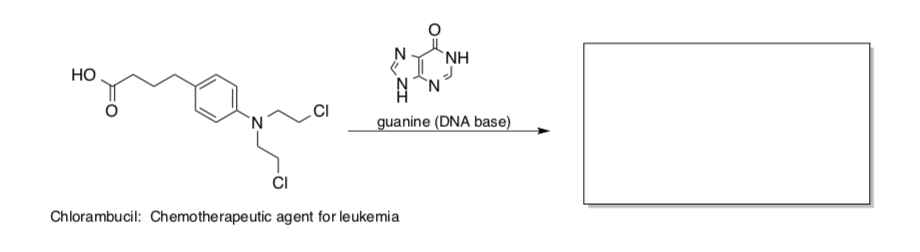
Treatment of tosylate 1 in acetic acid leads to substantial amounts of the rearranged acetate product, 2.
- Write a mechanism using arrow formalism for the formation of 2. This mechanism should explain the placement of substituents and stereochemistry.
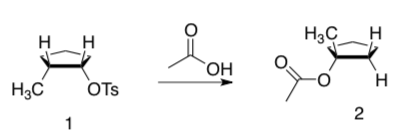
Stop...Summary Time! The SN1, SN2, E1, E2 Table
| # of steps | RDS | substrate | solvent | Nuc/Base | Temp | Stereochem | rearrangement | |
| SN2 | ||||||||
| SN1 | ||||||||
| E1 | ||||||||
| E2 |
Kinetic Isotope Effect for SN1 and SN2
Kinetic isotope effect (KIE) is the ratio of rate constants for a reaction run with a light (kL) isotope in the and the rate constant for the same reaction with a heavy (kH) isotope:
KIE = kL / kH
For the following two reactions:
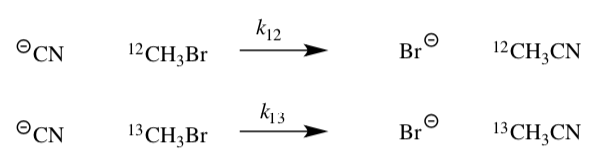
KIE= k12 /k13 = 1.082 ± 0.008
The kinetic isotope effect can help determine mechanism type.
Matsson, Dybala-Defratyka, Rostkowski, Paneth and Westaway, J. Org. Chem, 2005, 70, 4022-4027.
- Draw the two possible mechanisms for this reaction.
SN1:
SN2:
This isotope effect is different from the earlier example in the workbook because we are looking a different reaction than the nucleophilic substitution at a carbonyl.
In this case, small α-carbon KIEs near 1% are indicative of a carbocation reaction, while larger KIEs of up to 8% for a concerted substitution mechanism.
- For the KIE given for this reaction, which reaction is faster?
- Assuming that k13 has a rate of 100 s-1, what would the k12 rate be?
- Based on the KIE shown above, would you expect this reaction to be:
SN1 OR SN2
- Does that match what you know about this reaction? Explain.
Biological Alkylation in S-adenosylmethionine (SAM): SN1 or SN2?
In general, S-adenosylmethionine (SAM) is used in biological systems for the methylation of endogenous molecule.
SAM is a reactive methylating agent. It is the biological equivalent of MeI.
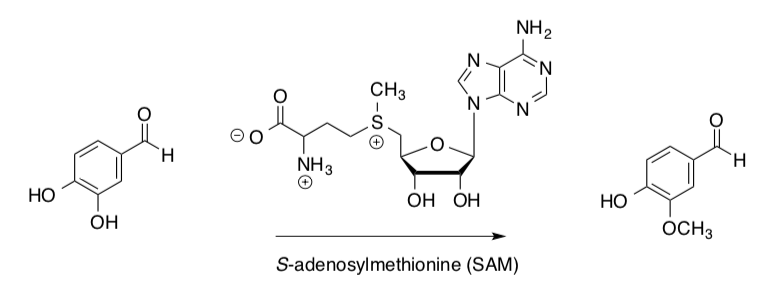
- For this substitution reaction:
- Identify the nucleophile.
- Identify the electrophile.
- Based on the structure of the carbon substrate would you predict this reaction to be?
SN1 OR SN2
Researchers found the k12 / k13 = 1.09 ± 0.05.
Schowen, et. al., J. Am. Chem. Soc., 1979, 101, 4359-4365.
- Based on the a-carbon KIEs, would you predict this reaction to be?
SN1 OR SN2
- Use curved arrows, to draw a mechanism for this reaction that includes your conclusion about the mechanism type.
Kinetic Isotope Effect for SN2 vs E2
Researchers have developed ways to study competition between two reactions in the gas phase by using mass spectrometry.
- Provide the products of each pathway.
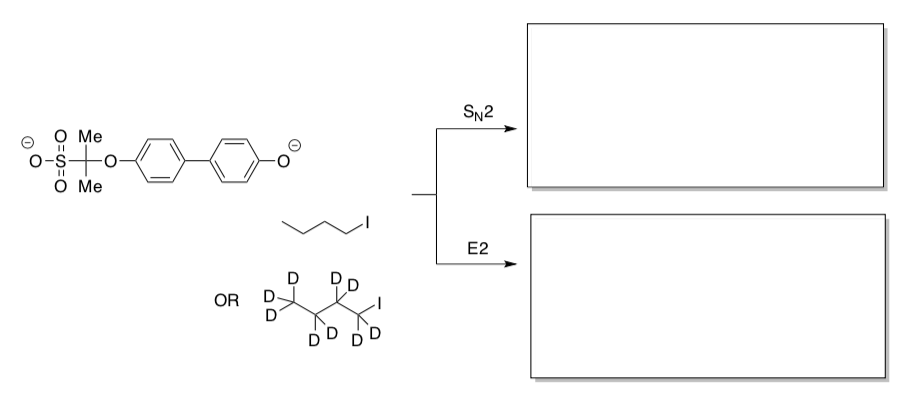
- Why do the researchers think these are SN2 and E2, rather than SN1 and E1?
The researchers probed “isotope effects” by running the reaction with regular iodobutane as well as deuterated iodobutane, in which the regular protium (1H) isotope of hydrogen (symbol H) is replaced by the deuterium (1H) isotope (symbol D).
- In a “primary isotope effect”, a C-H bond breaks up to seven times more easily than a C-D bond.
- In a “secondary isotope effect”, a C-D bond can make it slightly easier for the attached carbon to undergo geometric changes (or slightly harder, depending on the exact reaction).
- Would either of these effects be expected in an SN2 reaction? Why?
- Would either of these effects be expected in an E2 reaction? Why?
Researchers measured rate constants using iodobutane and its deuterated analogue. They observed kH/kD = 0.79 in the SN2 reaction and kH/kD = 7.2 in the E2 reaction.
- Compare these results to your predictions.
Predicting Major Reaction Type – Substitution or Elimination
Determining Major Reaction Type: Aliphatic Nucleophilic Substitution and b-Elimination
Practice Determining Mechanism and Product
- Use the flowchart to determine the mechanism type for each reaction.
- Draw the product formed (include stereochemistry!).
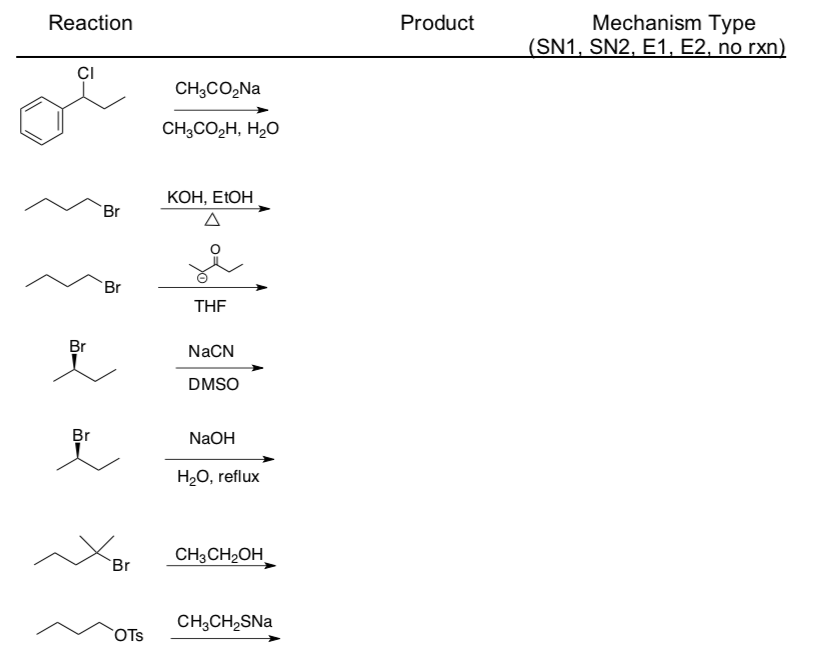
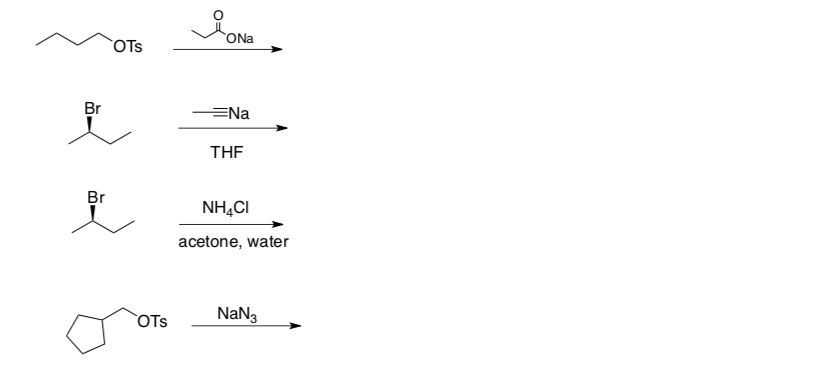
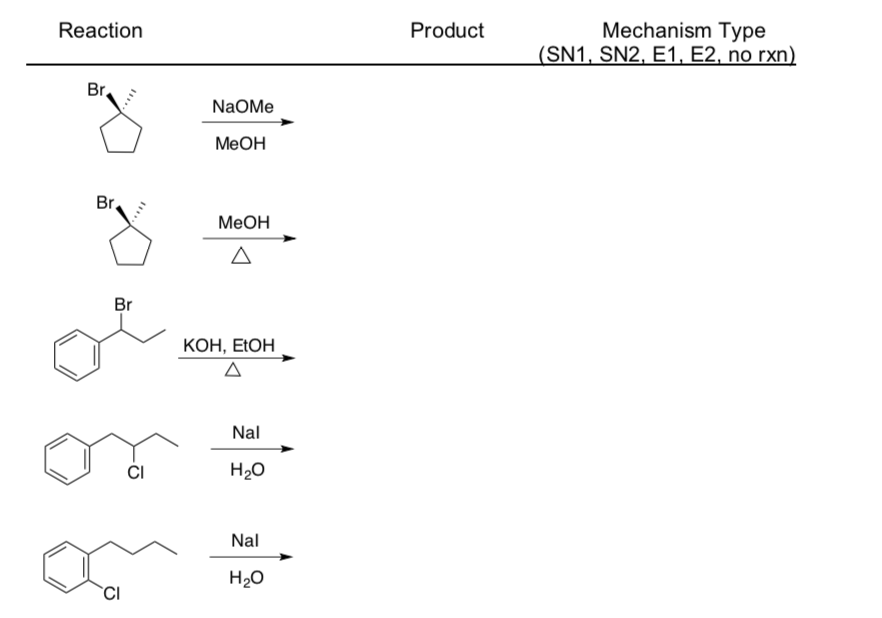
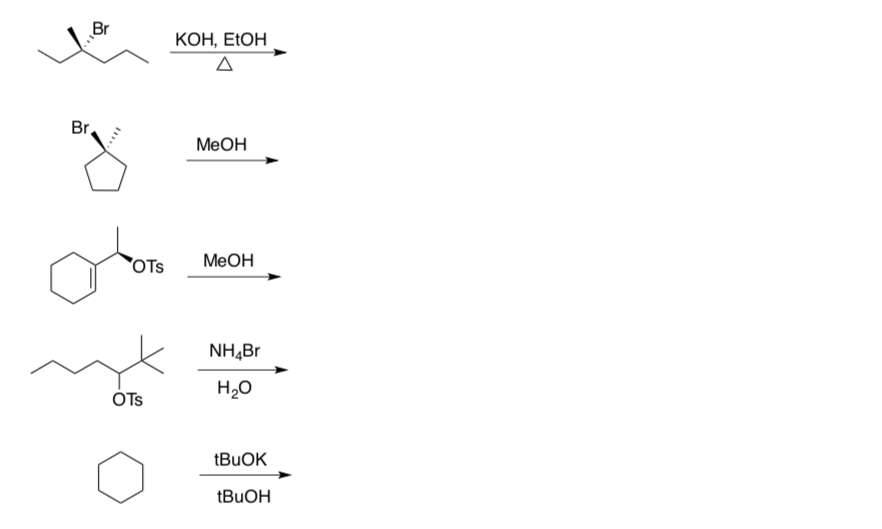

Substitution and Elimination Practice Problems
- EliminationandChairConformations
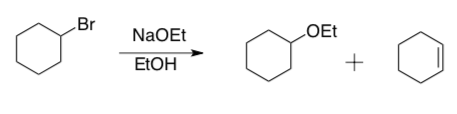
Since E2 and SN2 reactions both require a good Lewis Base, these reactions often compete. For example, when cyclohexyl bromide is treated with sodium ethoxide in ethanol, both cyclohexyl ethyl ether and cyclohexene are formed.
- Draw mechanisms using arrow formalism for the formation of both of these products.
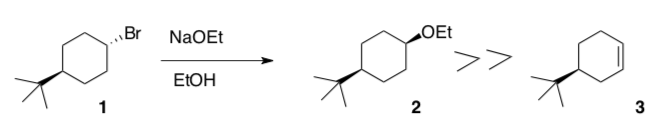
- When 1 is the starting material, the product is overwhelmingly 2. compound 3 is formed. Explain.
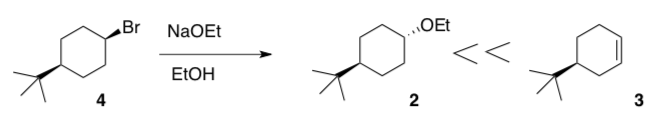
- When 4 is the starting material, the product is mainly 3. Explain.
- Draw mechanisms using arrow formalism for the formation of both of these products.
-
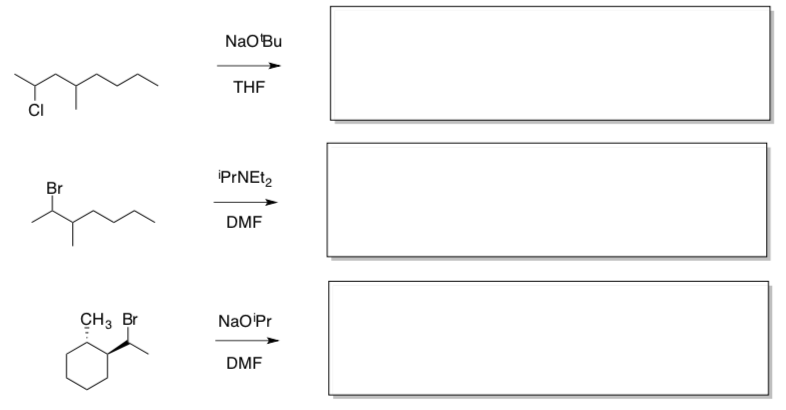
Predicting products or reagents
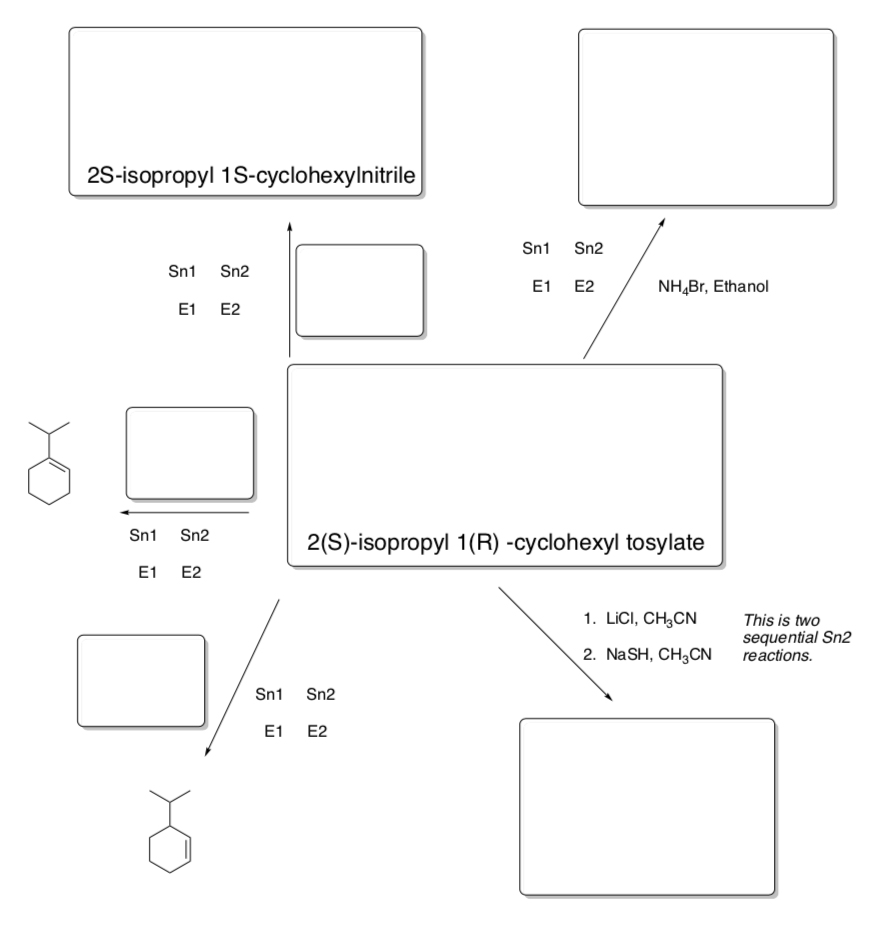
A student who started with 2(S)-isopropyl-1(R)-cyclohexyl tosylate was able to test it in reactions with different Lewis Bases.

- Draw the starting material with the correct stereochemistry.
- Circle the correct mechanism type based on the conditions.
- Fill in the box with a nucleophile/solvent/temperature that would maximize the desired product OR product given the solvent/nucleophile pair given.
- Practice Choosing Conditions
Here is a generalized reaction that can give multiple products.
LG= leaving groups Nu=nucleophiles Solvents T emperature OTs H- H2O hot CH3 Br- polar, aprotic cold F (CH3)3CO- non-polar I CH3ONa Green (no solvent) OH H2O D CH33OH 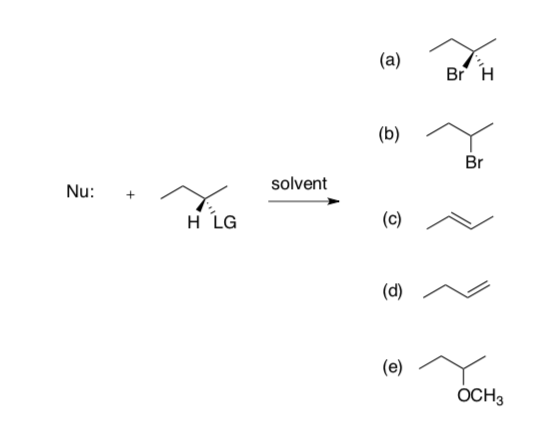
- For each product, pick from the table one LG, one Nu, a solvent and a temperature that would maximize the desired product.
- Very briefly explain your strategy.
- Even though hydride is a very poor choice for any of the reactions shown above, when LG = OH-, there is a rapid and irreversible reaction with hydride (H-). Explain what happens and provide a mechanism for this alternate reaction.
- Substitution and Elimination in competition:
- Explain carefully why reaction (a) fails but (b) succeeds:
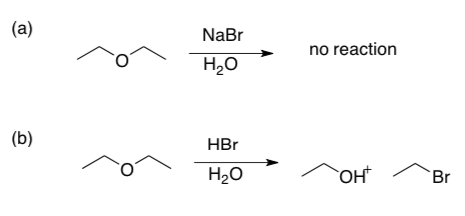
- Explain carefully why reaction (c) fails but (d) succeeds:
- In reaction (c), there is a product formed – what is it?
- Explain carefully why reaction (a) fails but (b) succeeds:
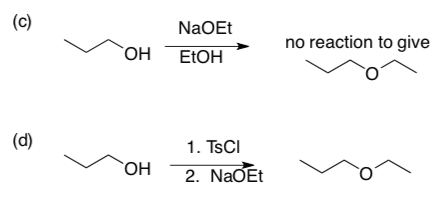
- Multistep reactions
- Predict the major product or reagent for these step. stereochemistry.
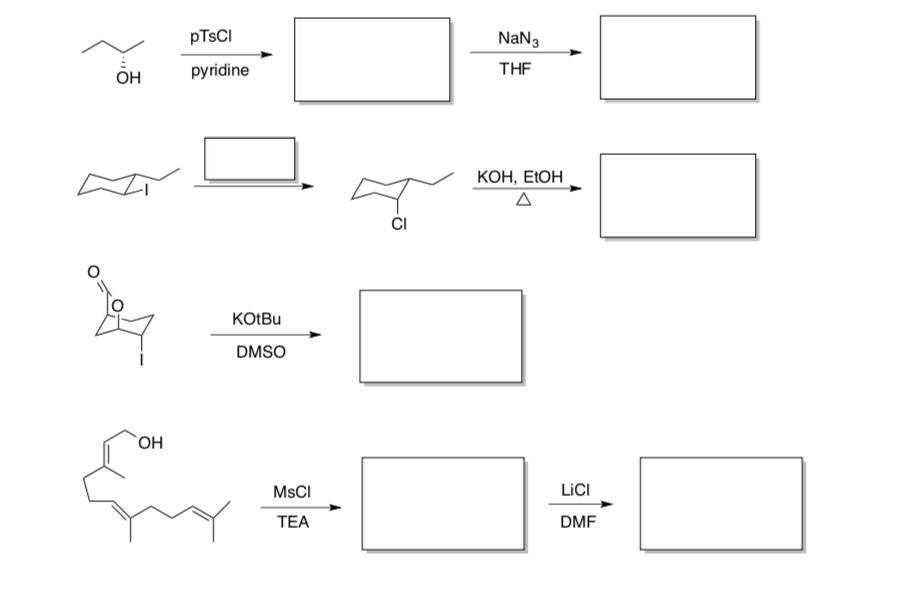
- Predict the major product or reagent for these step. stereochemistry.
- Recently a group of chemist sprepared the enantiomer of AZT(usedinthetreatment of AIDS) to see if it had different biological activity.
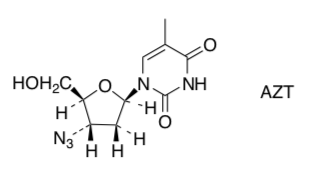
- Draw the enantiomer of AZT.
- Assign R/S configuration to all chiral centers of AZT.
- How many stereoisomers are possible for AZT?
- The following reaction is used in preparing AZT (below). What is the mechanism implied by the products obtained? Why?
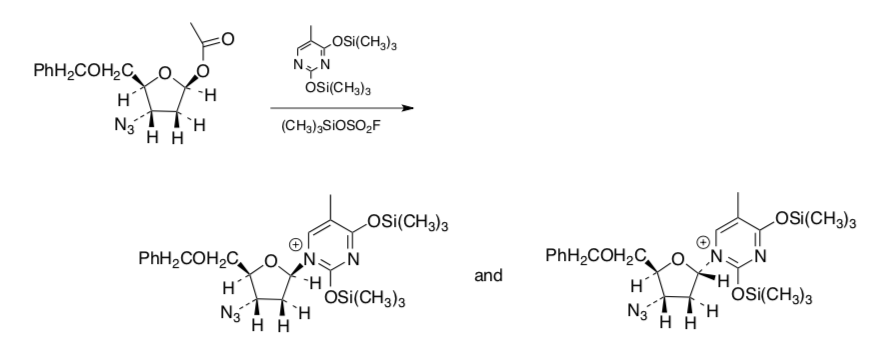
- Kinetics of Elimination
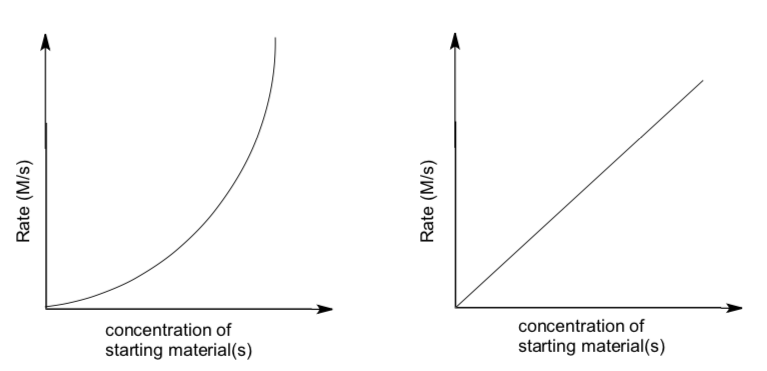
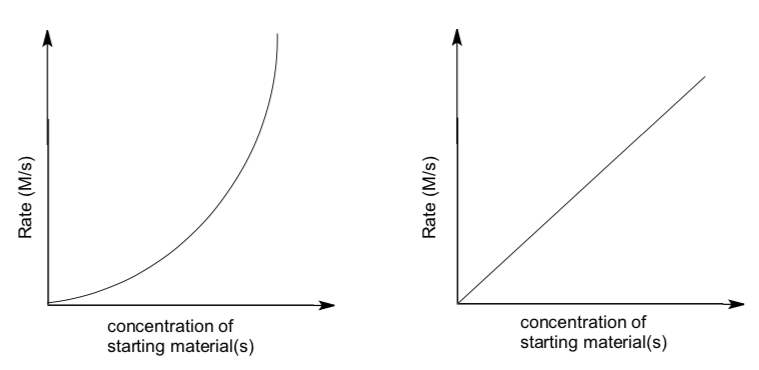
- Fill in the following graphs to show how the rates of E1 and E2 reactions would depend on reactant concentrations.
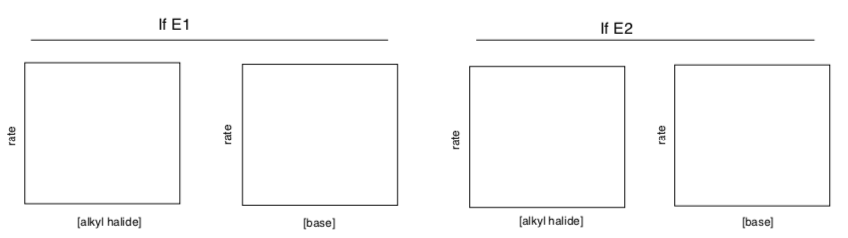
- Write rate laws for each case.
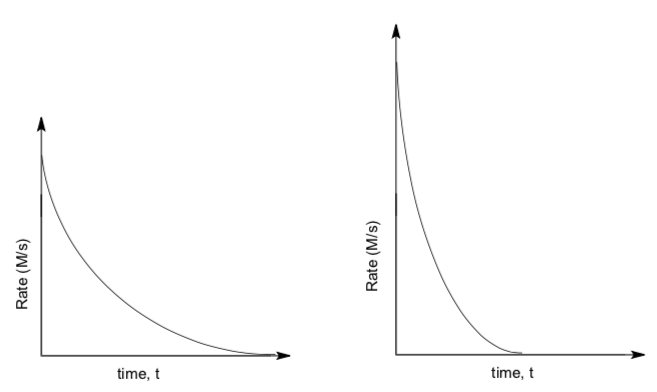
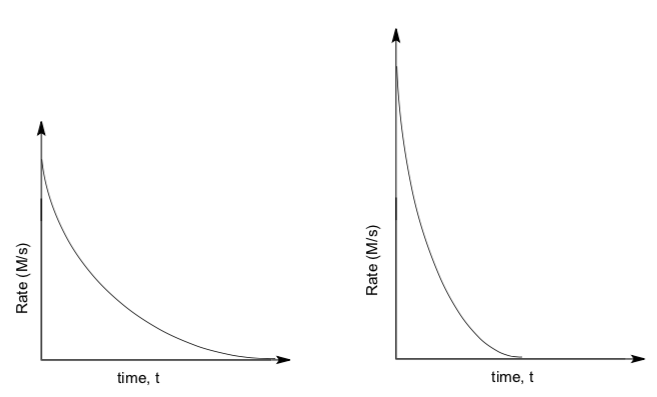
The normal course of an E1 and E2 reaction, starting out at the same rate, would look something like this:
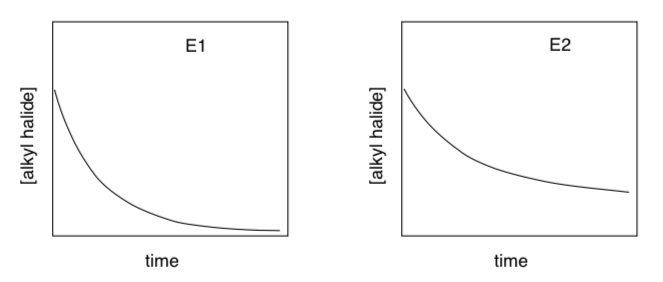
- Which reaction appears to finish sooner (i.e. the alkyl halide is all gone)?
- Why does one reaction slow down more than the other?
- Fill in the following graphs to show how the rates of E1 and E2 reactions would depend on reactant concentrations.
- Kinetics of Elimination (cont.)
The rate of the E1 reaction can be represented as -d[RX]/dt = k[RX] with RX = alkyl halide. This is called a differential rate law.
- A plot of -d[RX]/dt (i.e. Rate) vs. [RX] is linear. What is its slope?
If we integrate the expression, we get ln[RX] = -kt + ln[RX]0. Here, [RX]0 is the starting concentration of the alkyl halide. This is called the integral rate law.
- A plot of ln[RX] vs. time is linear. What is its slope? What is the y-intercept?
- Sketch the plot of ln[RX] vs time.
- Indicate on the graph the value of the slope and y-intercept.
The rate of the E2 reaction can be represented as -d[RX]/dt = k[RX][B] with B = base. If we start with equal concentrations of base and alkyl halide, [RX] = [B], then the rate law can also be written as -d[RX]/dt = k[RX][RX] = k[RX]2.
- In this case, is a plot of -d[RX]/dt vs. [RX] linear? If so, what is its slope?
- In this case, is a plot of -d[RX]/dt vs. [RX]2 linear? If so, what is its slope?
If we integrate the expression, we get 1/[RX] = kt + 1/[RX]0. Here, [RX]0 is the starting concentration of the alkyl halide.
- In this case, a plot of 1/[RX] vs. time is linear. What is its slope? What is the y- intercept?
- Sketch the plot of 1/[RX] vs time.
- Indicate on the graph the value of the slope and y-intercept.
Researchers often prefer to use these linear plots for kinetic analysis because of how easy it is to find the rate constant (just by finding the slope).
- Microsomal epoxide hydrolase
This enzyme converts epoxides into glycols. Researchers proposed two possible mechanisms:
- Enzyme as a Base. Draw arrows for this mechanism. Propose a structure for the product.
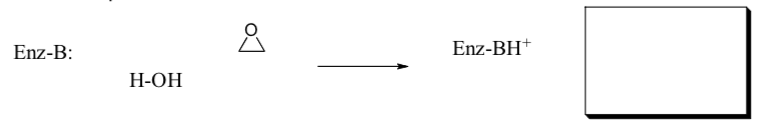
- Enzyme as a Nucleophile. Draw arrows for this mechanism. Propose a structure for the intermediate.
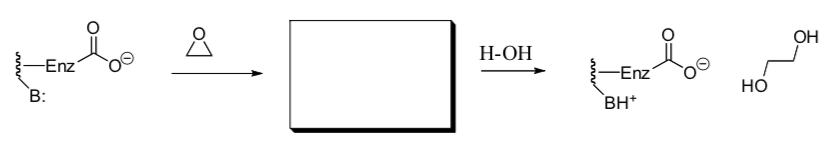
Researchers found that a single-turnover experiment in H2 18O, gave no 18O in the product diol.
- Which mechanism is consistent with this experiment? Explain.
If the enzyme contains aspartate labeled with 18O then the 18O becomes incorporated into the product.
- Which mechanism is consistent with this experiment? Explain.
- Enzyme as a Base. Draw arrows for this mechanism. Propose a structure for the product.
- Compound 5 is treated under the two sets of conditions shown below (A and B). Please note that 5 is not in an energy minimum form.
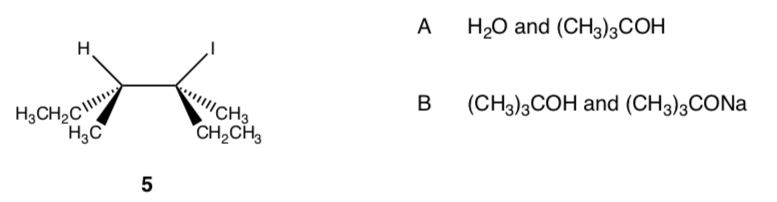
Under one set of conditions, the major product is X, a tetra-substituted alkene, and is the only tetra-substituted alkene formed. Under the other set of conditions, X is still formed, but a different tetra-substituted alkene Y and an alcohol Z is also produced.
Explain carefully what is going on. Provide structures of X, Y and Z, the set of reaction conditions (A or B) that leads to X or X, Y plus Z, and a mechanistic explanation including an arrow formalism for the product formation in the two cases.


