11: Association Diss
- Page ID
- 144001
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Ligand Exchange Mechanisms
Association and Dissociation Ligand Exchange

- Suggest rate laws for these two options.
- Draw a "potential energy diagram" for each option.
- Assume the y-axis is enthalpy.
- There is always an energy barrier for each step of the reaction.
- Use your judgment to decide on relative heights of these barriers.
Assume that breaking a sigma bond will be harder than a pi bond.
- Circle which step in each option will be the rds.

Lego Chemical Reaction Kinetics
Adapted from Cloonan, Nichol & Hutchinson, J. Chem. Educ., 2011, 88 (10), pp 1400–1403
Equipment
- Bag of small, interlocking, plastic brick building blocks– Two colors
- Watch or clock
The building blocks react according to the equation:
A + B ⇌ AB
Where A = Blue building block atom
B = Yellow building block atomAB = Molecule of blue and yellow atoms
AB = Molecule of blue and yellow atoms
- Watch or clock
Procedure Part 1: Synthesis Reaction Kinetics
- Record the total number of yellow blocks at start:_________
- Assign team roles. One participant will be the timer. One person will be the agitator (shake the box of blocks). Another participants will be an “Assembler.” One person will be the recorder.
- Agitator will shake the bag of Legos. Assemblers will reach into the bag and pull out two pieces without looking. If one blue atom and one yellow atom are selected, assemble them into a AB molecule by connecting the two building blocks perpendicularly. Put the molecule back into the box. If two of the same color atoms or any combination including an already assembled molecule are selected, put the atoms/molecules back in the box without changing anything. Repeat until the time limit of 1 min.
- The recorder will count the number of AB molecules created.
- Continue the synthesis reaction for at least three more intervals. After each minute interval, stop the reaction and count the number of molecules.



Procedure Part 2: Decomposition Reaction Kinetics
- Assemble all of the building blocks into AB molecules of one blue and one yellow building blocks.
- Record the total number of AB molecules at start:_________
- Assign Team roles again. One participant will be the timer and one person will be the agitator (shake the bag of blocks). The remaining participant will be “Disassembler.” And the recorder.
- Disassemblers reach into the bag and pull out one piece without looking. If an assembled molecule is selected, take it apart and put the separated blue and yellow atoms back into the box. If a single atom is selected, put it back into the box without changing anything. Repeat until the time limit. Count all of the
- Have the Disassembler continue the decomposition reaction for at least 3 more one-minute intervals. After 1 minute, stop the reaction and determine the number of atoms and molecules.

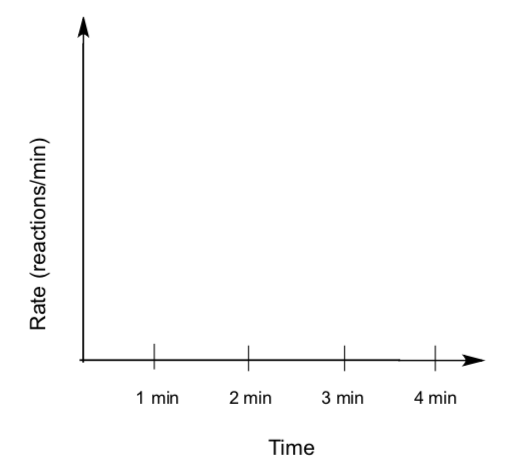

Conclusions about Lego Kinetics
- Was the reaction rate variable? If so, what did it depend upon?
- Was the synthesis or decomposition rate faster? Why? What factors were important?
- For your Lego reactions, decide the molecularity for each process.
Bimolecular Assembly
Unimolecular Dissassembly
- Which of these graphs best represents the unimolecular lego reaction? the bimolecular lego reaction?

- Which of these graphs best represents the unimolecular lego reaction? the bimolecular lego reaction?

Review: Ligand Field Theory Review*
High Spin/Low Spin
- Which metals (number of d electrons) differ in the order of octahedral orbital filling when placed in different field strengths: d____- d____
- Complexes with a strong field are often called:
Low spin High spin
- Complexes with a weak field are often called:
Low spin High spin
- Show a d5 high spin octahedral splitting diagram.
- Show a d6 low spin octahedral splitting diagram.
Metal Ion Effect
- For 3d metals (d4-d7): 3d metals can be high spin OR low spin. Larger charge results in __________ (high or low) spin.
- For 4d and 5d metals (d4-d7): In general, the size of Δo is __________ (larger or smaller) than for 3d metals. As a result, these complexes are typically _________ (high or low) spin.
*Answers for this LFT Review section are available on Canvas
Ligand Effects
These three factors have given three separate trends depending on the type of ligand (σ only, π acceptors, π donors).
- Label the three groups below as s only, p acceptors, p donors.
- en > NH3
- H2O > F- > RCO2 -> OH- > Cl- > Br- > I-
- CO, CN-, > phenanthroline > NO2 - > NCS-
- Place the three types of ligands on the arrow below based on the way they effect the Δo (spectrochemical series).

- Purely σ bonding ligands.
The stronger the Bronsted base, the ________ the splitting (Δo). A stronger base will bind more tightly to the metal. Thus the M-L σ orbitals will ____________in energy.
- Draw an entire MO diagram that explains this trend (review R1).
- π donating ligands__________splitting and cause a complex to be high spin.
Δ: H2O > F- > RCO2- > OH- > Cl- > Br- > I-
- Draw an MO diagram that explains this trend (review R1).
- π accepting ligands___________splitting and may cause a complex to be low spin.
Δ: CO, CN-, > phenanthroline > NO2- > NCS-
- Draw an MO diagram that explains this trend (review R1).
- Which type of octahedral complexes are more reactive to ligand exchange? a list of rules that will predict lability:
- eg (M-L sigma*) occupancy:
- d electron configuration
- d0
- d3
- d10
- size of charges:
- metal ion radius:
Magnetism
- How do you predict μso (approximate) for a complex using a splitting diagram?
- Explain how a measurement of μeff can provide evidence for the geometry of the complex.
UV
- How do the geometry and the type of ligand effect the color of a complex?
Jahn-Teller Distortions
Jahn-Teller theorem states that for a nonlinear molecule “there cannot be unequal occupation of orbitals with identical energy and the molecule will distort to remove the degeneracy and lower the energy of the complex.”
The Jahn–Teller effect is most often encountered in octahedral complexes of the transition metals. Such complexes distort along the z axis in order to lower the overall energy.

- What geometry is the complex starting to look like as the two axial ligands get further away?
- Compare the field splitting after the distortion for the field splitting of the geometry of your answer above.
- What happens to the overall energy of the distorted complex?
The distortion normally takes the form of elongating the bonds to the ligands lying along the z axis, but occasionally occurs as a shortening of these bonds instead.
Predicting the Jahn-Teller Effect
The Jahn–Teller theorem does not predict the direction of the distortion (compression or elongation). It only predicts when the geometry will become distorted.
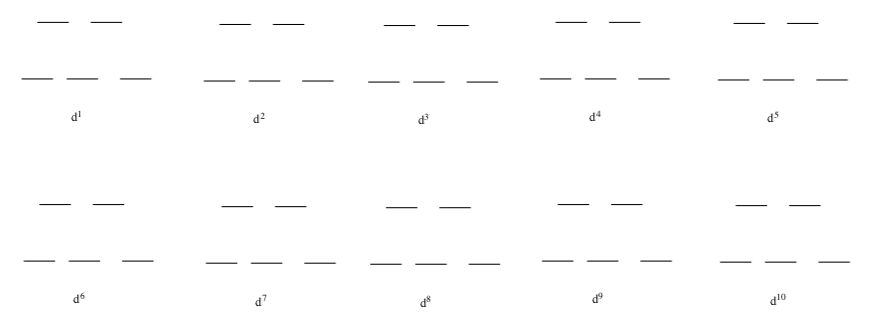
- Complete the high spin and low spin filling diagrams for octahedral complexes with d1-d10.
Low Spin:

High Spin:
- Which high spin complexes have unequally occupied t2g orbitals?
- Which low spin complexes have unequally occupied t2g orbitals?
- Which high spin complexes have unequally occupied eg orbitals?
- Which low spin complexes have unequally occupied eg orbitals?
- Based on your filling diagrams, explain the trends in the following table:
- Which type of unequally occupied orbitals gives weak distortions?
- Which type of unequally occupied orbitals gives strong distortions?
# of d electrons 1 2 3 4 5 6 7 8 9 10 high spin Jahn-Teller w w s < w w s low spin Jahn-Teller w w w w s s w = weak Jahn-Teller effect
s = strong Jahn-Teller effect
blank boxes = no Jahn-Teller effect
Summary: Jahn-Teller Effect
The Jahn–Teller theorem predicts when the geometry will become distorted.
- Review your filling diagrams for octahedral complexes with d1-d10.
- Which high spin complexes have unequally occupied t2g orbitals?
- Which low spin complexes have unequally occupied t2g orbitals?
- Which high spin complexes have unequally occupied eg orbitals?
- Which low spin complexes have unequally occupied eg orbitals?
- Based on your filling diagrams, explain the trends in the following table:
- Which type of unequally occupied orbitals gives weak distortions?
- Which type of unequally occupied orbitals gives strong distortions?
- What does a Jahn-Teller distortion indicate about lability of the ligand?
Applications of the Jahn-Teller Theorem
- The bond lengths of [Cu(NO2)6]-4 have been experimentally determined. The axial bonds are 2.31 pm and the equatorial bonds are 2.05 pm. Note that the “octahedral’ coordination of Cu+2 is not really octahedral. It is said to be Jahn- Teller distorted.
- Draw this compound showing the differential bond lengths.
- Draw the ligand field splitting diagram for this complex.
- Explain why it is distorted.
- The crystal structure of CrF3 shows that the chromium is in an octahedral hole surrounded by 6 fluorines all with a distance of 190 pm. However, MnF3 has the same basic crystal structure but the Mn-F has bond lengths of 179, 191 and 209 pm. Explain these differences.
Association / Dissociation
One ligand in a transition metal complex is replaced by another ligand.

- Draw the possible mechanisms for the ligand substitution.
Associative:
Dissociative:
- Label the two mechanisms above with molecularity.
Challenge:
- Draw a third possible mechanism for the ligand substitution where the bond-making and bond-breaking steps occur simultaneously. This is called Ia(Associative Interchange).
- What would the reaction potential diagram look like?
- What would the molecularity for this reaction be?
Factors Affecting Ligand Substitution Mechanism Type
Transition metal complexes have flexible geometry / coordination environment, so predicting a mechanism can be difficult. However, there are some general observations.
- Electron count
The order of steps may be influenced by electron saturation at the metal.
- Explain how the electron count in an 18-electron complex may influence the order of steps in a ligand substitution.
- Explain how things may be different in a 16-electron complex.
- Draw square planar (NH3)2PtCl2.
- Count the electrons on the metal in the complex.
- Show a mechanism with arrows to replace one of the amines with PH3.
- Electron Filling
Complexes containing metals such as Cr+3 or Mo+3 are often resistant to association.
- Draw the complex and provide an octahedral ligand splitting diagram for Cr(acac)3 (acac is a bidentate anionic ligand).
- Using the splitting diagram, explain why this complex may not be willing to bind another ligand.
- Geometry
The order of steps may be influenced by the geometry at the metal.
- Four coordinate complexes of Ni2+ and Pt2+ often have two different geometries. What is the geometry of each? Why?
- Which of these two metal ions might be more likely to undergo substitution via association followed by dissociation: (NH3)2NiCl2 or (NH3)2PtCl2? Why?
- Sterics
Order of steps may also be influenced by crowding at the metal.
- Draw the tetrahedral complex, (PPh3)4Pd, and provide an electron count for the metal in the complex.
- Show a mechanism with arrows for the replacement of a triphenylphosphine ligand with a tricyclohexylphosphine ligand.
- Metal Lability
The order of steps may be influenced by the lability of the metal.
- Which of these two metal ions might be more likely to undergo substitution via dissociation followed by association: L6V2+ or high spin L6Fe2+? Show why using a d orbital splitting diagram.
- Which of these two metal ions might be more likely to undergo substitution via association followed by dissociation: L6Ti4+ or L6Cr3+? Show why using a d orbital splitting diagram.
- Ligand Lability
The order of steps may be influenced by the coordinating ability of the ligand.
- If a ligand is labile, the complex would be more likely to undergo an ( associative / dissociative ) mechanism.
Assume that the field splitting corresponds to coordination strength.
- Rank the three types of ligands (sigma donor, pi donor, pi acceptor) in order of the spectrochemical series.
- Which of these two ligands might be more likely to undergo substitution via dissociation followed by association? Explain.
- Ligand type: H2O vs. CO
- π donor strength: I- vs. Cl-
- σ donor strength: NH3 vs. NEt3
- Jahn-Teller
Jahn-Teller distortions can lead to increased lability and, hence increased ligand exchange, in some complexes.
- Explain why [Cr(H2O)6]2+ undergoes substitution more easily than [Cr(H2O)6]3+.
Pre-dissociation
Sometimes a ligand may leave to make room for substitution of a different ligand, then return after the substitution occurs.
- What is the electron count on the osmium in the octahedral (PMe3)4Os(H)Cl?
- What is the probable order of steps in the substitution mechanism?
Replacement of the anionic chlorine ligand takes place via the mechanism below.
- Provide curved arrows to keep track of electronic movement and bonding changes.

- Provide an explanation for why the reaction steps occur in this order.
- Provide curved arrows to keep track of electronic movement and bonding changes.
Summary of Associative/Dissociative Ligand Substitution Reactions
- Explain how these factors affect Association and Dissociation Reactions
- Sterics
- Electron filling
- Geometry
- Electron Count
- Metal Lability
- Ligand Lability
- Jahn-Teller Distortions
- Draw reaction progress diagrams for association and dissociation.
- Draw graphs of rate vs. concentration for a unimolecular and a bimolecular reaction.
Association and Dissociation Practice Problems
- Based on the data below, answer the following questions.
Rate constants (relative) for water replacement Rate constants (relative) for water replacement Entering ligand [Cr(H2O)6]3+ [Cr(NH3)5(H2O)]3+ NCS- 180 4.2 NO3- 73 - Cl- 2.9 0.7
Br- 1.0 3.7 I- 0.08
- CF3COO- - 1.4 Adapted from Miessler and Tarr, Inorganic Chemistry, 4th ed, Prentice Hall, 2011.
- Does the rate constant vary depending on the incoming ligand?
Cr(H2O)6]3+ (circle one): varies little variation
[Cr(NH3)5(H2O)]3+(circle one): varies little variation
- Based on the ligand dependence, predict the mechanism of ligand exchange:
Cr(H2O)6]3+ (circle one): associative dissociative
[Cr(NH3)5(H2O)]3+(circle one): associative dissociative
- Does the rate constant vary depending on the incoming ligand?
- The rates of water substitution in [M(H2O)6]2+ complexes involve the exchange of “bulk” water molecules for those that are bound to the transition metal as ligands. When compared to other first row metals in the +2 oxidation state, Cu+2 and Cr+2 exchange water molecules more than 10 times faster than other ions in this row. Explain this trend.
- Below are some data for stepwise replacement of water molecules with ethylenediamine, en ( en = H2N(CH2)2NH2). For example, K1 represents the equilibrium constant for the reaction shown below:
[M(H2O)6]2+ + en -> [M(H2O)4(en)]2+ + 2 H2O
K2 represents the replacement of the next two water molecules. K3 represents the last two water molecules.
K1 K2 K3 Cu+2 3 x 1010 1 x 109 0.1 (estimated) Ni+2 2 x 107 1 x 106 1 x 104 - K>1means:
Products are favored A mixture is formed Reactants are favored
- K1 and K2 are both large regardless of metal. Explain why denticity and field strength play a role in these equilibrium constants.
- Explain why K3 for Cu2+ is unusually small. Consider Jahn-Teller effects and electron count.
- K>1means:
Association and Dissocation Practice PSA
Part 1. Ligand Substitution Mechanisms
- Draw a curved arrow mechanism for an associative ligand exchange for the reaction above.
- Draw a reaction profile diagram for this reaction.
- Draw a curved arrow mechanism for a dissociative ligand exchange for the reaction above.
- Draw a reaction profile diagram for this reaction.
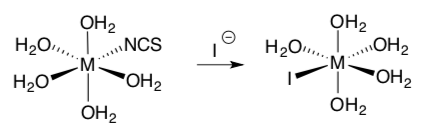
Part 2. Studies on the ligand exchange were done to determine the type of mechanism.
Sun, Zhang, Ji, Hartsock and Gaffney, J. Phys. Chem. B., 2013, 117, 12268-12275.

Kinetic data from the reaction when the central ion (M) is Ca2+
| [I-] | [NCS-] | k |
| 7M | .35M | 75 ps-1 |
| 11M | .35M | 130 ps-1 |
- What type of exchange is occurring for Ca+2?
Associative Dissociative
Kinetic data from the reaction when the central ion (M) is Mg+2
| [I-] | [NCS-] | k |
| 6M | .24M | 82 ps-1 |
| 11M | .24M | 75 ps-1 |
- What type of exchange is occurring for Mg+2?
Associative Dissociative
- Explain why the type of ligand exchange might be different for these two ions.
Part 3. Implications regarding the difference in exchange mechanisms.
Sarcoplasmic reticulum (SR) Ca2+ transport has a central role in regulating cardiac function in health and disease. The cardiac SR is an intracellular membrane that actively maintains cytosolic Ca2+ concentration during contraction–relaxation.

An exchange process of Mg2+ with Ca2+ provides a possible modulatory function of Mg2+ on SERCA activity. Crystal structures indicate that Mg2+ ions bind in one of the two Ca2+ binding sites, yet freely exchange with Ca2+ ions when they are both present in the cytosol.
- Thinking about the ligand exchange mechanisms, suggest a reason why the Mg2+ is readily exchanged out of the binding pocket.
Trans Effect of Square Planar Complexes
Trans Effect
Langford and Gray, Ligand Substitution Processes, 1996, W.W. Benjamin, Inc.
http://authors.library.caltech.edu/2...ngford_Lsp.pdf
In a ligand substitution involving square planar complexes, the ligands on the starting material direct the position of the incoming ligand.
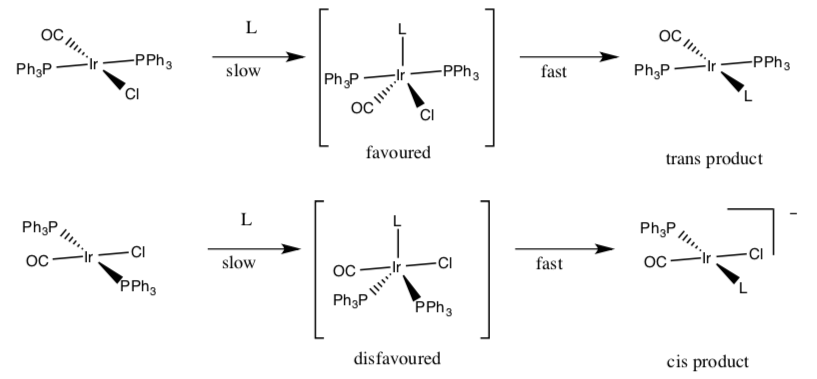
For example, if bromide were to replace chloride in Vaska’s complex (below), there are two possible products.
Ligand Substitution Example:

Effect of π–acceptor ligands
A strong π–acceptor ligand will have a strong trans effect -- it will increase the rate of substitution of the trans ligand.
Order of trans effect for π–acceptors: C2H4 > CN- > CO > NO2
- In Vaska’s complex (above), circle the strongest π–acceptor ligand.
- The ligand opposite the π–acceptor ligand will become more labile. Which ligand is opposite the p–acceptor ligand?
- If the bromide replaces the ligand opposite the π–acceptor ligand, predict which product will be favored. Circle one.
A or B
- Square planar compounds typically undergo..... Circle one.
Associative Dissociative
MO of p–acceptors Ligands in Trans Effect
The role of π–acceptors in the trans effect depends on stability of the trigonal bipyramidal intermediate.
- Redraw the favored intermediate below, with CO on the left.
- Use Br- as the incoming ligand.
- Keep the PPh3 ligands 180° apart.

If we add the orbitals cartoons on this same axis, we can see an extend MO overlap from the incoming ligand, the metal d orbital and the CO ligand.
- Label all of the ligands in the cartoons below.

Conjugated pi interactions are always more stable with orbitals in one plane.
http://authors.library.caltech.edu/2...ngford_Lsp.pdf
To understand why the other approach is NOT favored, we will repeat this analysis for the other possible trigonal bipyramidal intermediate.
- Draw the disfavored isomer.
- Use Br- as the incoming ligand.
- Keep the PPh3 ligands 60° apart.

- Add the MO cartoons on these axes.
- This MO overlap does NOT stabilize the intermediate as much as the picture on the previous page. Explain.
- Hint: Orbital overlap is the strongest when the orbitals are all in one plane.
Trans Effect with s–donor ligands
There is also a trans effect for strong sigma donor ligands. This effect is typically not as strong as the π–acceptors ligands.
But the effect is the same.
- The ligand trans to a strong σ–donor ligand will be more [ labile / inert ] and will be exchanged. Circle one.
Order of trans effects for sigma donors follows:
H- > CH3- > SR2 > PR3 >I- > Br- > Cl- > py > NH3 > H2O > HO- > F-
It is thought that σ–donors can have a strong trans effect because of competition for the metal acceptor orbital. The following cartoon shows the results of overlap between a strong s donor and a metal and a weak s donor and a metal.
- In each box, draw a ligand and its donor orbital trans to the ligand already shown.
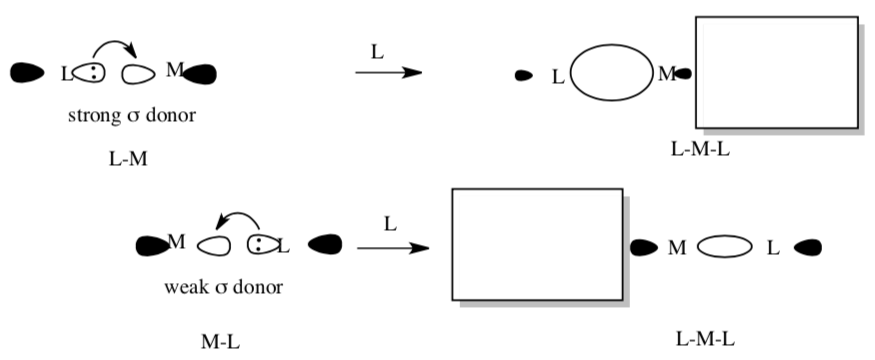
- Comment on the extent of orbital overlap with the metal in each case.
- Comment on the metal-ligand bond strength of a ligand trans to a strong σ donor.
- Comment on the metal-ligand bond strength of a ligand trans to a weak σ donor.
Trans Effect Practice Problems
- Cis-platin [cis –(NH3)2PtCl2] is a powerful anticancer drug (used for testicular and ovarian cancer).
- Fill in the products of the following reaction schemes.
- Indicate which route would be a better approach to making cis-platin.

- Predict the products in this reaction.

- Predict the products in this reaction.

- Provide a two-step synthesis for trans-[PtCl2(NO2)(NH3)]1- starting with [PtCl4]2-.
- Provide a two-step synthesis for cis-[PtCl2(NO2)(NH3)]1- starting with [PtCl4]2-.
- Even though most square planar complexes show a dependence on the incoming ligand, there can be a dependence on the leaving group as well.
[Pt(dien)(X)]+ + pyridine –> [Pt(dien)(pyr)] + X
X (leaving group) Rate constant NO3- Very fast H2O 1900 Cl- 35 Br- 23 I- 10 SCN- 0.3 NO2- 0.05 CN- 0.02 - Explain why there might be a dependence on the leaving group as well as the incoming group. What property of the leaving groups might help explain the order of the rate constants for substitution?
Summary of Trans Effect
- Circle the best descriptors in the following sentences:
- A ligand trans to a (strong / weak) σ donor will be lost more easily than a ligand trans to a (strong / weak) σ donor.
- Ligands that are (strong / weak) σ donors have strong trans effects.
- Good π–acceptors have (strong / weak) trans effects.
- Provide a list of the ligands in order of their trans effect.
- Draw pictures that show how the orbital alignment of a pi donor to M to pi acceptor favors one intermediate over the other.
It is thought that σ–donors can have a strong trans effect because of competition for the metal acceptor orbital.
- Use the MO cartoons to explain the following trend for trans effect of σ–donors:
I- > Br- > Cl- >F-


