10: Using Kinetics to Determine Mech
- Page ID
- 144000
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Rate Determining Step
The rate of a reaction with several mechanistic steps is often determined by the slowest step, known as the rate-determining step (RDS). After that step is finished, everything else can happen very quickly.
In a reaction coordinate, the transition state with the highest energy is the rate- determining step of a given reaction.
- In the following reaction profiles:
- Label the Ea for each step
- Label the Ea for the overall reaction
- Label the rds for each reaction.

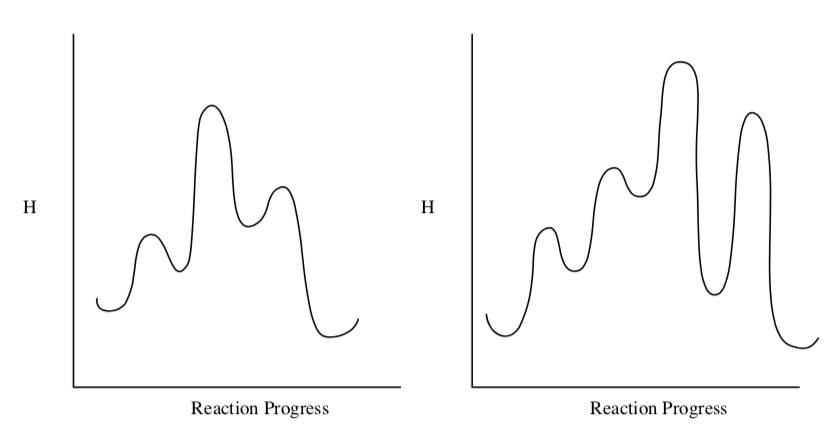
Using Kinetics to Look at Mechanisms
Assume you don't remember anything about the mechanism of this reaction (it was last semester, after all). There are 2 possible mechanistic pathways.
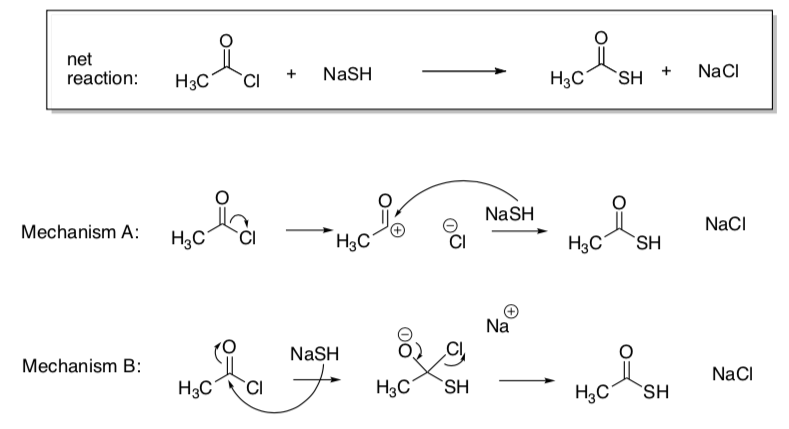
- Draw a "potential energy diagram" for each option.
- Assume the y-axis is enthalpy.
- There is always an energy barrier for each step of the reaction.
- Use your judgment to decide on relative heights of these barriers.
Assume that breaking a sigma bond will be harder than a pi bond.
- Circle which step in each option will be the rds.

Rate Laws and Molecularity
The rate law defines the change in concentration of product in terms of a "rate constant", k, and the concentrations of species that are needed in order to get to the rate-determining step. Species that come along after the rate-determining step don't affect the rate.
For example,
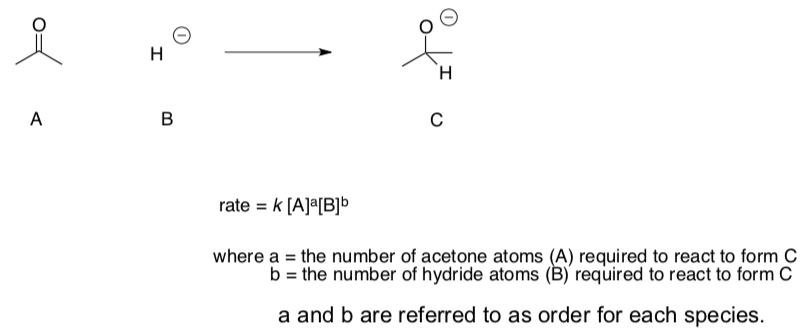
- What is the order of this reaction with respect to acetone?
- What is the order of this reaction with respect to hydride?
- Write a rate law for this reaction:
rate =
Molecularity is the number of colliding molecules or atoms that are involved in the rate determining step.
- What is the molecularity of the reaction above?
Unimolecular OR Bimolecular
Unimolecular and Bimolecular reactions are fairly common.
Termolecular reactions are rare.
- Provide a reason why termolecular reactions are not likely.
More Information on Rate Laws
For the reaction rate law:
$$
\text { Reaction Rate }=\mathrm{k}[\mathrm{A}]^{2}[\mathrm{B}]^{\mathrm{b}}
\]
Chemists refer to the sum a + b as the kinetic order of a reaction.
- Complete the table by determining the reaction order for each of the reactions.
Table 1 below shows some experimental rate laws for several chemical reactions.

- What is the reaction order with respect to each reactant in the first reaction in the table?
CH3Br ______________
OH— ______________
- What is the reaction order with respect to each reactant in the second reactionin the table?
(CH3)3CBr ______________
OH— ______________
- For the third reaction in the table, does the reaction order have anything to do with the coefficients in the balanced equation? Explain why or why not?
- For the fourth reaction in the table:
$$
\text { rate }=k[\mathrm{Cl^-}][\mathrm{OBr^-}][\mathrm{OH^-}]^{-1}
\]if [Cl-] increases, rate will ___________
if [Cl-] decreases, rate will ___________
if [OH-] increases, rate will ___________
if [OH-] decreases, rate will ___________
- For the fourth reaction in the table, the reaction is inverse first order in hydroxide ion. What do you think that means?
Summary of Rate Laws
- Define:
- Rate determining step:
- Rate law:
- Reaction order:
- Answer the following regarding rate orders as either True or False.
- True OR False: To find the rate order (i.e. exponent) for each reactant you just need to look at the balanced equation and make the order of each reactant equal to the coefficient in the balanced equation. No experiments are needed.
- True OR False: To find the rate order (i.e. exponent) for each reactant you need to perform several experiments where the concentration of each reactant is systematically changed. The relative rates are then examined to determine the value of the reaction order.
- Choose the correct statement(s) from the list below.
- The rate order for a reactant must equal the coefficients in the balanced equation.
- The rate order of a reactant must be a whole number that is positive.
- The rate order of areactant must be non-zero.
- The rate orders are determined by what is happening at the microscopic level and cannot be determined without experimental data.
Kinetics to Determine Mechanism
Back to this reaction again:
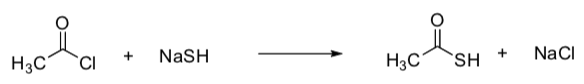
- Draw the 2 possible mechanisms.
- OptionA
- OptionB
- Suggest rate laws for these two options.

Three Tools to Study Mechanism
- In a fictional study, Bunsen and Beaker measured rates for the reaction using different starting concentrations of reactants. They compiled the following data.
[CH3(CO)Cl]0 (mol/L)
[NaSH]0 (mol/L)
d[CH3(CO)SH] / dt (mol/Ls)
0.10 1.0 1.5 x 10-4 0.10 2.0 2.9 x 10-4 1.0 0.10 1.6 x 10-4 2.0 0.10 3.1 x 10-4 - When the concentration of acid chloride is doubled, what happens to the rate? What does that tell you about the order with respect to the acid chloride?
- When the concentration of NaSH (nucleophile) is doubled, what happens to the rate? What does that tell you about the order with respect to the nucleophile?
- Which mechanistic option and corresponding rate law (from above) fits the data?
Mechanism A Mechanism B
- Bunsen and Beaker also carried out some high-level quantum mechanical calculations on the acid chloride reactant.
- Populate the electrons and label the HOMO and LUMO levels in the Hückel MO diagram for ethanoyl chloride.

- When a nucleophile donates electrons to this molecule, which orbital will the electrons go into?
- What will happen to the bond order as a result?
- Which mechanism has a pi bond broken in the rds? Circle one.
Mechanism A Mechanism B
- Does this data confirm or refute your previous conclusions about the mechanism?
- Populate the electrons and label the HOMO and LUMO levels in the Hückel MO diagram for ethanoyl chloride.
- Bunsen and Beaker also looked the difference between the regular 12C rate and the labeled 13C rate. This often suggests a change in geometry or hybridization at the labeled carbon during the reaction.
- Is there a geometry change during the rds in Option A?
- Is there a geometry change during the rds in Option B?
Bunsen and Beaker very carefully measured the rate of reaction of 13C-labeled ethanoyl chloride, CH3(CO)Cl. They found the ratio of rates, k 12C : k 13C = 0.91 (+/- 0.02).
- Does this ratio indicate that a geometry change occurred?
- Which rate law (from above) fits the data?
Mechanism A Mechanism B
- Does this data confirm or refute your previous conclusions about the mechanism?
Summary of Kinetics to Determine Mechanism
- Explain the 3 ways to determine whether a reaction is unimolecular or bimolecular.
- Measuring Order:
- MO Calculations:
- Kinetic Isotopic Effect:
- For a reaction with the rate law of rate = k[A][B].
- Describe what will happen to the rate if the concentration of A is doubled AND the concentration of the B is doubled.
- For a reaction with the rate law of rate = k[A][B].
- Describe what will happen to the rate if the concentration of A is doubled AND the concentration of the B stays the same.
- For a reaction with the rate law of rate = k[A]2[B].
- Describe what will happen to the rate if the concentration of A is doubled AND the concentration of the B stays the same.
Application Problems:
- Consider the following reaction:
2N2O5(g) ⇌ 4NO2(g) + O2(g)
rate = k[N2O5]m
Table 2 shows some experimental data for the reaction from above.
[N2O5] Initial Rate Experiment 1 0.101 M 14 x 10-4 M/s Experiment 2 0.202 M 28 x 10-4 M/s - Why does it make sense that in general reactions go faster when more reactant is present? (Imagine that you are small enough to see atoms and molecules.)
- From Table 2, what effect did doubling the amount of N2O5 have on the rate? What would tripling the amount of N2O5 likely do?
- Since the rate and concentration move in the same proportion (i.e. doubling the N2O5 doubles the rate), determine if the exponent m is 0, 1, or 2.
rate = k[N2O5]m
- Does the exponent in the rate law match the coefficient in the balanced equation?
- The following graph shows concentration vs. time data for a certain reaction.

- Is E a product or reactant? Explain.
- At which point is the reaction faster, A, B, or C?
- The reaction 2Fe2+(aq) + 2H+(aq) + H2O2(aq) à 2Fe3+(aq) + 2H2O(l) is first order with respect to Fe2+, zero order with respect to hydrogen ion, and first order with respect to hydrogen peroxide. Write the rate law AND give the overall order.
Rate law:
Overall Order: ____________
- Consider a chemical reaction between compounds A and B that is 2nd order in A and 1st order in B.
- Write the rate law.
- Fill in the blanks in the chart.
[A]o as M [B]o as M Initial Rate of Formation of C as M/min 0.20 0.05 0.011 0.05 0.044 0.40 0.088
- Consider the following reaction:

- Write the equation for the overall reaction
- Identify any reaction intermediates
- What is the molecularity of each step?
- The reaction below occurs in a single mechanistic step. Write the rate law for this reaction.
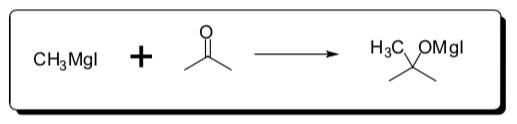
- Examine the reaction below:

There is an alternative reaction mechanism in which the bromine bond is formed at the same time as the chlorine bond is broken.
- The first step of this reaction is relatively slow due to the instability of the carbocation intermediate and the second step happens very fast. Which step will determine the rate of reaction?
- What reactant(s) will the rate depend on?
- Propose a rate law for the given reaction.
- Draw a mechanism for this alternate pathway.
- Propose a rate law for this alternate pathway.
- Practice PSA: Esterification of Trifluoroacetic Acid

- The reaction of trifluoroacetic acid yields the following ester upon reaction with an alcohol.
- Show the mechanism (include the role of the H+).
- The H+ emerges unchanged at the end of the reaction.
- What is the role of the proton?
- Kinetic data are provided for this reaction.
[Alcohol] M [TFA] M Initial Rate M/s [Alcohol] M [TFA] M Initial Rate M/s 0.186 0.5 1200 0.1 1.5 1950 0.186 1.0 2500 0.2 1.5 4200 0.186 2.0 4800 0.3 1.5 6100 - Provide a rate law for the reaction based on the data.
- Graphical representation of rates.
- Fill in the following graphs that represent information about this reaction.
- Fill in the following graphs that represent information about this reaction.
- This reaction displays the following temperature dependence.

- Explain the temperature dependence using collision theory.
- The rate of this reaction is also dependent upon substrate structure.

- Draw the structures of the alcohols included in the table.
- Explain the rate dependence on their structures.
- The reaction of trifluoroacetic acid yields the following ester upon reaction with an alcohol.


