17: Multinuclear Spin Over Half
- Page ID
- 332817
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Multinuclear NMR: Nuclei with spins > ½
Nuclei with a spin > ½ that have a small enough electric quadrupole moment that lines are fairly sharp, or at least observable.
2H, 7Li, 6Li, 11B, 14N, 17O, 33S
In this unit, we will look spectra of some nuclei that have spin > ½ to understand the interpretation of the coupling.
Spin States
When a nucleus has a spin of ½, then there is the possibility of 2 states. However, if the nucleus has a spin that is larger, then there are more than two states.

- Draw the possible spin state for a nucleus with a spin of 2.
- Draw the possible spin state for a nucleus with a spin of 5/2.
This will impact the splitting patterns!
Coupling to nuclei of spin = 1
To determine the multiplicity, you can use the formula:
\[k = 2 n l + 1\]
l is the spin
n is the number of nuclei
Rather than Pascal's Triangle, we can use the Trinomial Triangle to determine line shape for nuclei of spin = 1:

NMR Analysis of Heteronuclear NMR with Spin > ½*
* All spectra are either from SDBS (Japan National Institute of Advanced Industrial Science and Technology) or simulated.
Deuterium in 13C NMR
Deuterium has a spin = 1 and the coupling 1JCD = ~30 Hz.
This is the spectrum for the common NMR solvent, CDCl3. When you zoom in it is a triplet.

- Using the formula (k = 2 n I +1), explain coupling pattern for the carbon of CDCl3 in the carbon spectrum.
- Compare the line shape of this peak to triplets that you have seen before.
Deuterium in 1H NMR
The germinal coupling is often small, 2JHD = 1-5 Hz.
- When you use 99% CD2Cl2 as the solvent for proton NMR, you see a small peak from the 1% that is CHDCl2. Thus, the proton in CHDCl2 will show coupling to the deuterium. What splitting pattern will you see?
When you use d6-acetone, there is a small quantity of d5-acetone present. Thus, the proton will appear in the 1H NMR and will show coupling to the deuterium.
- Predict the coupling in acetone d5. What will the line shape be?

Boron NMR
Boron has two naturally occurring NMR active nuclei. Both nuclei have spins of greater than ½ and are quadrupolar. 11B has a spin of 3/2 and 10B is spin 3.
11B is more sensitive so we will look at a few of these spectra.
J. Chem. Ed., 1977, 54 (8), 469-473.
The simplest system in which 11B and 1H nuclei are coupled is the borohydride anion, BH4-1. All four protons are magnetically equivalent and have a spin of ½.
- The 11B NMR spectrum is thus a symmetrical __________________ using 2nl + 1 for coupling with four equivalent protons. Keep in mind that the pattern for the coupling is based on the nucleus that is coupled to the atom observed. Thus, in 11B NMR, we use the spin of the 1H atoms coupled to the observed boron atom.
- The pattern is a ratio of __________________ .
11B NMR spectrum of NaBH4

1H NMR spectrum of NaBH4.
All four protons are magnetically equivalent, so they appear as one peak split by the boron nucleus.
- The 1H NMR spectrum is __________________ using 2nl + 1 and the spin of the 11B nucleus is 3/2.

Boron NMR (cont.)
Coupling from boron-proton is small so 1JBH is seen. No 2JBB is seen.
The spectrum of B4H10 in 11B NMR.
- Looking at symmetry, how many peaks (unique borons) will be observed in this structure.

- Analyze the coupling in this spectrum for this of B4H10.
- Assign the peaks.
- Indicate the atoms to which they are coupling.
- What are the two coupling patterns?
- Show a splitting diagram.

Practice Multinuclear NMR
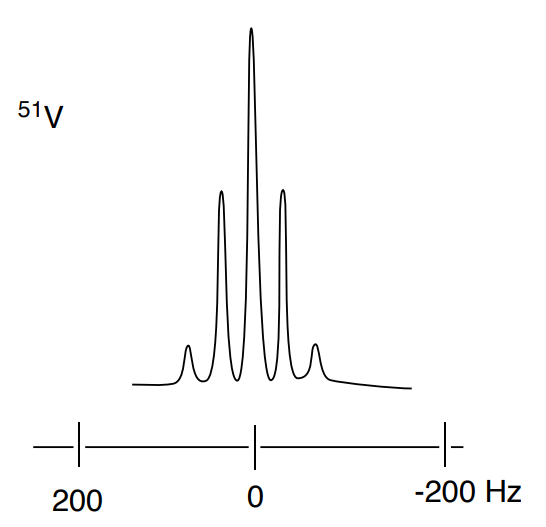
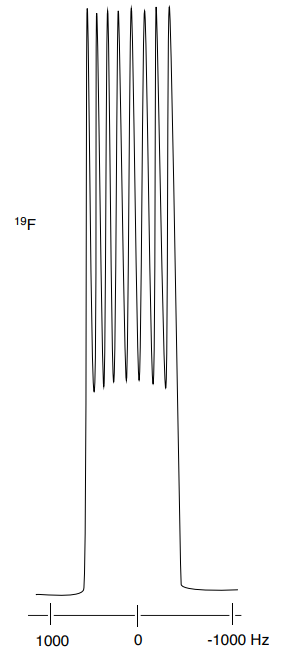
Determine the structure of the ion [VOF4]- using the coupling shown in the 51V and the 19F spectra below.
- What is the spin (I) of 51V?
- What is the spin (I) of 19F?
- What is the structure of the ion? Include the VSEPR geometry. Explain your reasoning.