16: Multinuclear
- Page ID
- 332816
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Multinuclear NMR* Nuclei with Spin = 1/2
NMR is not limited to 1H and 13C nuclei. There are lots of other nuclei that can be studied using NMR.
NMR spectra of nuclei with a spin quantum number of ½ follow the same kind of coupling behavior we see in 1H NMR. We will look at some other NMR active nuclei in this section.
NMR can also be performed with nuclei that have spins larger than ½ (next section).
To be useful in NMR, a nucleus must have a non-zero spin. There are also a few other factors to consider:
1. Isotopic Abundance. Remember, elements have a variety of isotopes and each isotope has a different spin quantum number. Some spin active nuclei such as 1H are 99.9% abundant, but others such as 17O have such a low abundance (0.037%) that no signal is seen in a typical sample.
- 13C is only 1.1% abundant. Explain why we need to use a lot of sample (and/or more scans) to obtain a 13C spectrum versus a 1H spectrum.
2. Sensitivity. The extent to which one orientation (energy state) is favored over the other depends on the strength of the small nuclear magnet. Thus, some nuclei are less sensitive. Some sensitivities are so low, such as 103Rh (100% abundant but only 0.000031 sensitivity).
- The lower the sensitivity, the ____________the amount of time and ____________ sample it will take to get a signal.
3. Nuclear quadrapole. For spins greater than 1/2, the nuclear quadropole moment is usually larger and the line widths may become excessively large. This can sometimes be overcome by running the sample at low temperature.
There are reference tables of Nuclear Spin, Sensitivity, and Natural Abundance. (META: missing link)
*All spectra in this unit are simulated or used with permission.
Common Nuclei Used in NMR
For nuclei with high natural abundance and spin = ½, spectra are easy to obtain, and are routinely used by chemists and biochemists.
Nuclei with spin = 0 have no NMR properties except for isotope shifts on nearby nuclei: 16O, 12C, 32S.
Nuclei with a spin = ½ are easily observed, give sharp lines and have easily observed J coupling.
100% abundance: 1H, 19F, 31P, 169Tm, 103Rh, 89Y
1-50% abundance: 13C, 119Sn, 117Sn, 77Se, 29Si, 207Pb, 125Te, 195Pt, 111Cd, 203Tl, 205Tl, 113Cd, 199Hg, 129Xe
Less than 1% abundance: 15N (0.37%), 3H (0%)
In this unit, we will look 13C, 31P and 19F spectra because these are routinely used for analysis of compounds with phosphorus and fluorine compounds.
13C-1H Coupling in 13C NMR
There are 3 possible types of 1J coupling in an organic molecule:
- C-H coupling in hydrogen NMR
- C-C coupling in carbon NMR
- C-H coupling in carbon NMR
- Why are the first two types of 1J coupling not typically observed?
The 13C spectra that we have seen so far are proton decoupled. If the spectra are not decoupled, the carbons are split by the n+1 rule. These are 1 bond coupling interactions, so the J values are quite large, typically 100 - 250 Hz.

Here is the aliphatic region of the 13C NMR spectrum of ethyl phenylactete with and without decoupling.

When the peaks are closer together than the example above, the J values are often larger than the difference between carbon environments.
- Why are most 13C spectra proton decoupled?
31Phosphorus NMR
The ordinary range of chemical shifts range from about d 250 to -250 ppm.
31P NMR is often obtained with proton decoupling.
- Suggest a reason.
The 1H decoupled 31P spectrum of diethyl phosphonate:


Typically, the phosphorus carbon couplings do not appear.
- Suggest a reason.
31Phosphorus NMR in Structures with Phosphorus-Phosphorus Coupling
Phosphorus atoms can couple to each other just as we saw coupling in proton NMR spectra.
Typical Coupling Constants:
1JPP = 150-700 Hz
2JPP = 5-20 Hz
3JPP = 1-10 Hz
The 31P {1H} NMR (proton decoupled) spectrum of a square planar complex [MH(PPh3)3] is shown below.

- How many unique P atoms are present in this structure?
- Assign the atoms to the peaks and explain the splitting pattern seen in this spectrum.
31Phosphorus-1H Coupling in 31P NMR
A proton NMR spectrum that is not decoupled will show JHP.
The typical ranges of phosphorus-hydrogen coupling constants:
1JPH = 150-700 Hz
2JPH = 5-20 Hz
3JPH = 1-10 Hz
This is the 1H-coupled 31P spectrum of diethyl phosphonate.
- The spectrum shows one peak, a doublet of pentets. Draw a splitting diagram for this coupling pattern.
- Draw double-headed arrows on the structure indicating the couplings.

Practice 31P Spectra
- Predict the coupling pattern in the 31P {1H} NMR of tetramethylphosphonium.
- Predict the coupling pattern in the 31P NMR (without decoupling) of the same compound.

31Phosphorus Coupling in the 1H NMR
Of course, the phosphorus atoms will also couple to the hydrogen nuclei in a typical proton NMR. The coupling constants are the same.
- Analyze the 1H NMR spectrum of diethylphosphonate shown below. Assign the peaks and the coupling patterns.

Practice with Phosphorus Coupling in 1H NMR
1. 1H NMR of a square planar complex [MH(PPh3)3] was performed. The hydride (H attached to the metal atom appears in the region from - 4.76 to - 5.24 ppm.
An expansion of this region of the spectrum is shown below

- Draw a splitting diagram to explain the splitting pattern seen in this spectrum.
- Indicate which splitting comes from which atoms.
NMR of Phosphorus Containing Biological Molecules
As so many biological molecules contain phosphoryl groups, it is worthwhile to look at the different nuclei NMR of these molecules.
Consider the case of isopentenyl diphosphate, the building block molecule used by cells to make isoprenoid compounds such as farnesyl groups.
1H NMR of IPP

Image from: Kaneda, Kuzuyama, Takagi, Hayakawa & Seto. An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces sp. strain CL190. PNAS, 2001, 98 (3) 932-7.
- Why is the H1’ peak a td?
13C NMR of IPP
- In the 13C NMR, C1 shows up at 67.0 ppm as a (d, 2J = 7.2 Hz) in the proton decoupled 13C NMR spectrum and C2 appears at 40.7 ppm (2J = 4.0 Hz). Explain this coupling pattern.
31P NMR of IPP
- Explain the 31P NMR spectrum:
- 11.03 (d, JPP = 20.0 Hz)
- 7.23 (d, JPP = 20.0 Hz)
19F NMR Spectroscopy
Isotope Fluorine-19 is 100% abundant, has a spin of 1/2, and is almost as sensitive as proton.
2JFH = 40-60 Hz
3JFH = 6-50
4JFH = 1-5
The 19F NMR spectrum of fluoroacetonitrile is shown below.
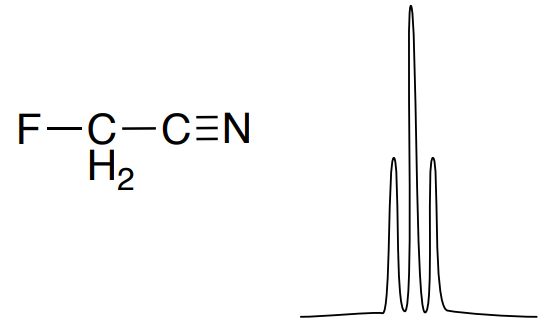
- Explain the coupling pattern.
- Predict the coupling constant.
Carbon-Fluorine Coupling in 13C NMR
13C NMR spectra are not usually fluorine-19 decoupled, so fluorine couplings appear in 1D 13C NMR spectra.
1JCF = 245 Hz
2JCF = 21
3JCF = 8
4JCF = 3
- Assign the peaks and the coupling constants in the carbon spectrum of trifluoroethanol (CF3CH2OH).
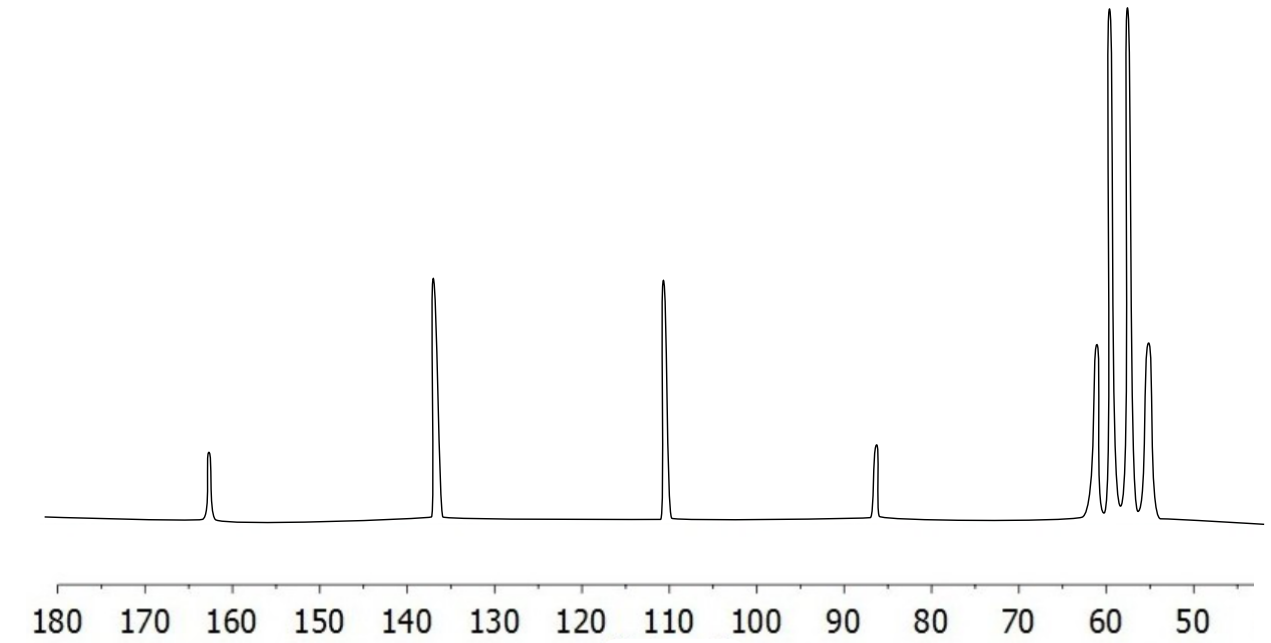
- Assign the peaks and the coupling constants in the carbon spectrum of 1,4 difluorobenzene.

Proton-Fluorine Coupling in 1H NMR
Coupling between hydrogen and fluorine is very strong.
2JFH = 40-60 Hz
3JFH = 6-50
4JFH = 1-5
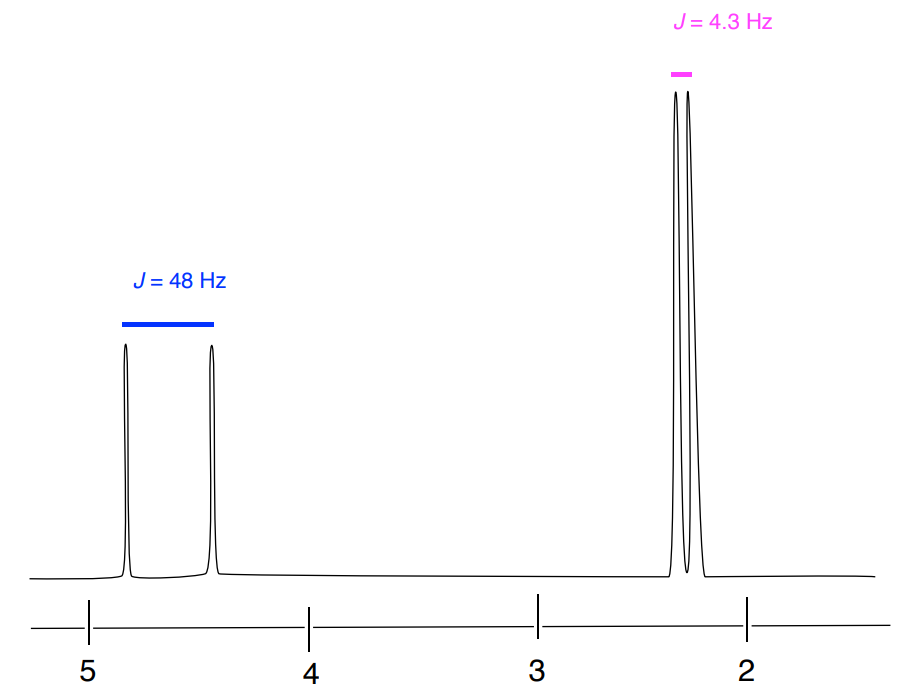
- Assign the peaks and explain the coupling in the proton spectrum of fluoroacetone.
- Fluoroacetonitrile shows only a doublet in the 1H NMR spectrum.
- Explain the splitting pattern.
- Predict the coupling constant for this peak.
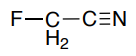
NMR of Multiple Isotopes that have Nuclei with Spin = ½
Some elements (e.g. Sn, Te) have more than one NMR active nucleus, and so several sets of peaks (“satellites”) are observed.
Tin is unique because it has three NMR active spin nuclei, 115Sn (0.35%), 117Sn (7.6%), and 119Sn (8.6%).
Tin NMR is mostly used for the study of organotin compounds but is also applicable to inorganic tin compounds.
A simple structure such as trimethyltin chloride yields the following 1H NMR spectrum.

The three methyl groups appear as a singlet when bonded to the non-NMR active Sn nucleus. However, as you zoom in closer you can see that there are also separate peaks for the methyls coupling to the three NMR active Sn isotopes. They all have slightly different shifts.
- Why are these peaks so small relative to the methyls next to non-NMR active nucleus? Hint: Look at the abundances.
- Draw the splitting diagram for all three doublets.
- Measure the coupling constants for the three isotopes:
- 2J119SnH :
- 2J117SnH :
- 2J115SnH :



