Chemistry in Biology
- Page ID
- 50672
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Chemistry has been defined as the science that is concerned with the composition, properties, and structure of matter and with the ways in which substances can change from one form to another. But this definition is too broad to be useful. Chemistry isn't the only science that deals with the composition and transformations of matter. Matter is also composed of cells, which transform by meiosis or mitosis, or of organs which are transformed by disease, or of organisms which are transformed by ageing. These are normally considered the subject of Biology. Chemists are unique because they understand or explain everything, even the subjects studied by biologists, in terms of the properties of just over 100 kinds of atoms found in all matter, and the amazing variety of molecules that are created by forming and breaking bonds between atoms. So chemistry is defined by its approach, not its subject matter. Chemistry explains or understands any subject in terms of the properties of atoms and molecules.
Chemistry provides a unique perspective that is particularly important in Biology. Since genetics and nucleic acid chemistry were combined in the second half of the twentieth century into the new field of genomics, a virtual revolution has occurred. It started with Watson and Crick's 1953 Nature article [1], which explained genetic reproduction in terms of intermolecular bonding in "the double helix".
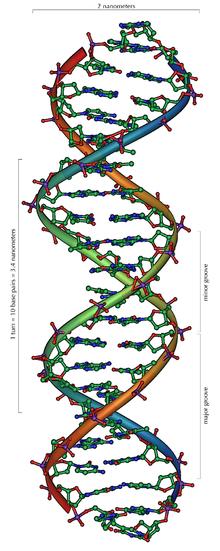
The union of the biological perspective, with its genetic explanation of inheritance of physical traits, with a chemical understanding of the molecular structure of DNA [2], led to rapid change in virtually all of science. New approaches were born in medicine (where genetic manipulation has led to new drugs and approaches to healing), in forensics (where the Polymerase Chain Reaction is used to generate DNA evidence), and in genetics, where DNA can be used to trace evolutionary changes.
The interplay of chemistry and biology is evident in contemporary brain chemistry. Biologists have discovered that on the cellular level, Fresh neurons arise in the brain every day, but that (in rats) learning enhances the survival of new neurons in the adult brain. The more engaging and challenging the learning, the greater the number of neurons that stick around [3]. It seems, then, that a mental workout can buff up the brain, much as physical exercise builds up the body. At the same time, chemistry has helped understand how neurons interact through neural transmitters whose molecular shape is critical to their function, so the modes of understanding complement one another.
Critter Chemistry is a feature of an American Chemical Society Journal that explains biological effects observed on the organism or organ level characheristic of biology, in terms of properties of the molecules that constitute the organism. For example, geckos can cling to walls not because of some macroscopically observable ‘stickiness’, but in terms of a type of intermolecular bond (van der Waals forces) explained by chemists.[4]
Chemistry provides an understanding of the molecular structure of amino acids and how they bond to make proteins. This helps explain amino acid deficiency diseases like kwashiorkor, that biologists have characterized, and helps nutritionists design healthy diets for vegetarians, athletes, and new mothers. Dietary fiber, which reduces heart diseases and other health problems, actually describes a chemical property, rather than the physical property suggested by the word "fiber". A physical property is one which is not explained at the molecular level: fibers are observed and characterized with simple microscopy. Dietary fiber does not refer to threadlike structures, but rather to non-digestible foods. It includes cellulose (a polymer of sugars), hemicellulose (a version of cellulose with a shorter polymer), pectin (“glue” that binds plant cells together), lignin (a constituent of plant cell wall). “Dietary fiber” may be colloidal (invisibly small clumps of molecules), as in clear Metamucil®. The indigestibility is a chemical property, since molecular bonds must be broken (chemical changes) to transform molecules as they are digested. Finally, biologists often are called upon to explain that a chemical is not necessarily non-organic; it is merely a molecular species that is understood in terms of the properties of the atoms or molecules that constitute it. Biomolecules in "natural foods" are no different than synthetic molecules made in the lab; they are both "chemicals" when understood in terms of their molecular structure or properties. When people speak of "chemical free" products, they usually mean that the material is found in nature and so was not designed by a chemist, even though the molecules designed by chemists are identical. Chemists often say 'everything is a chemical" because it is possible to understand everything in terms of molecular properties.
From ChemPRIME: 1.0: Prelude to Chemistry
References
- ↑ A Structure for Deoxyribose Nucleic Acid Watson J.D. and Crick F.H.C., Nature 171, 737-738 (1953)www.nature.com/nature/dna50/archive.html
- ↑ en.Wikipedia.org/wiki/DNA
- ↑ Tracey J. Shors, "Saving New Brain Cells," Scientific American, March 2009, pp. 47-48.
- ↑ : http://pubs.acs.org/cen/critter/critterchemistry.html
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


