Silicones 5. Organic Silicones at General Electric
- Page ID
- 2923
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Heat and Chemical Resistant Silicone Rubber
The invention of practical silicone resins occurred at General Electric. Silicones came from a combination of patient thought and brilliant laboratory technique. And they came from a willingness to be led by others' ideas. In the late 1930's, Corning chemists made samples of silicone materials that insulated critical electrical devices at up to 180 degrees Celsius. But the insulators could not be manufactured efficiently at a cost that was practical.
They would be impossibly difficult to make in high volume and be outlandishly costly. But Corning made a decision to invite the people over at nearby General Electric to drive over to look at samples of their new resin. The Corning scientists at Corning, NY and the General Electric scientists at Schenectady still visit each other regularly. The relationship between the two research groups has been strong ever since Corning developed the machinery to begin automatic manufacture of glass globes for GE's light bulbs.
So in 1938, Corning's Dr. James Hyde invited scientists in GE's research group come over to Corning for a presentation about Corning's new high temperature insulating materials, the silicones. GE's Winton Patnode made the visit to Corning; he listened to the Corning story and it confirmed some ideas of his own. Dr. Patnode had believed that silicon containing compounds would indeed provide the answer to the high-temperature insulation problem. He drove back to Schenectady and sat down with a friend doing insulation research at the General Electric laboratory, Dr. Eugene G. Rochow.
Let's look at what happened next beginning with Dr. Rochow's internal monologue. Dr. Rochow was trained as an inorganic chemist, a chemist whose experience with organic chemistry - the chemistry of carbon compounds, was quite limited.
"I was busy with several . . . problems at the time, but kept thinking about Patnode's (ideas) and Hyde's silicone. The questions and answers went like this.
(Q) What is really needed?
(A) A flexible inorganic insulation that will stand at least 200 or 300 degrees Celsius in service.
(Q) What are the chances of its being 100% inorganic
(A) Practically nil.
(Q) If we have to admit some of the enemy (carbon-containing groups) into the camp, what form should it take?
(A) It should constitute an absolute minimum of the whole and it should have no carbon-carbon bonds, lest it end up as C-C-C-C-C conducting resins in the motor.
(Q) How should these organic groups, so grudgingly admitted and only for the sake of flexibility, be attached to the inorganic main framework of this imaginary new polymer?
(A) By direct carbon-metal bonds, not through oxygen because they would always be hydrolyzable (react with water - editor) under damp conditions.
(Q) What would be the best metal for the backbone?
(A) Considering that we shall be dealing with carbon-metal bonds, probably silicon, because its alkyls such as \((CH_3)_4Si\) are thermally stable and do not react with water or air in the 200-300 degrees Celsius range."
From: Silicon and Silicones, Eugene G. Rochow, Springer-Verlag, Berlin, 1987, p.65
Rochow's thoughts led to a single structure. All his considerations led to a dimethyl silicone polymer:
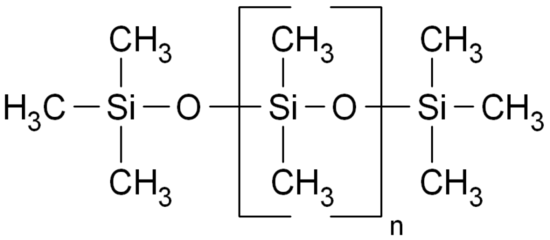
The decision to seek a methyl silicone took Rochow in a different direction than Kipping had gone. But Rochow recognized he would meet one serious problem with the methyl silicones. They had never been made and he could find no guidance in the chemical literature for how he would make them.
![]()
And Eugene Rochow said, "So what? Get busy and make it!"


