Extra Credit 12
- Page ID
- 82922
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)17.2: Galvanic Cells
Q17.2.1
Write the following balanced reactions using cell notation. Use platinum as an inert electrode, if needed.
- \(\ce{Mg}(s)+\ce{Ni^2+}(aq)⟶\ce{Mg^2+}(aq)+\ce{Ni}(s)\)
- \(\ce{2Ag+}(aq)+\ce{Cu}(s)⟶\ce{Cu^2+}(aq)+\ce{2Ag}(s)\)
- \(\ce{Mn}(s)+\ce{Sn(NO3)2}(aq)⟶\ce{Mn(NO3)2}(aq)+\ce{Sn}(s)\)
- \(\ce{3Cu(NO3)}(aq)+\ce{Au(NO3)3}(aq)⟶\ce{3Cu(NO3)2}(aq)+\ce{Au}(s)\)
S17.2.1
For these problems it is important to keep in mind that you must determine the proper reactants and products at both the anode nd the cathode and keep track of the states of these elements. In addition, it is necessary to be familiar with electrochemical cell notation and terms:
- Cellular notation is read from left to right
- The left denotes the anode and the right the cathode (these are referred to as half-cells)
- Anode: The anode is where the oxidation reaction takes place. In other words, this is where the metal loses electrons.
- Cathode: The cathode is where the reduction reaction takes place. This is where the metal electrode gains electrons.
- | separates different phases (a comma is used to differentiate between species of the same phase)
- || is used to differentiate between the anode and cathode, representing the salt bridge
- Concentrations are often written in the parentheses after the phase notation (the lack of concentrations denotes standard conditions, ex. 1M)
When solving these problems we will use a three step process:
Step 1: Write the two half-reactions.
Step 2: Identify the cathode and anode.
Step 3: Construct the Cell Diagram.
Q1. \[\ce{Mg}(s)+\ce{Ni^2+}(aq)⟶\ce{Mg^2+}(aq)+\ce{Ni}(s)\]
Step 1: Write the two half-reactions.
\[\ce{Mg}(s)⟶\ce{Mg^2+}(aq)\]
\[\ce{Ni^2+}(aq)⟶\ce{Ni}(s)\]
Step 2: Identify the anode and cathode.
The anode is the \(\ce{Mg}(s)\) since it increases in oxidation state from 0 to +2.
The cathode is the \(\ce{Ni^2+}(aq)\) as it decreases in oxidation state from +2 to 0.
Step 3: Construct the Cell Diagram.
\[\ce{Mg}(s)│\ce{Mg^2+}(aq)║\ce{Ni+}(aq)│\ce{Ni}(s)\]
Q2. \[\ce{2Ag+}(aq)+\ce{Cu}(s)⟶\ce{Cu^2+}(aq)+\ce{2Ag}(s)\]
Step 1: Write the two half-reactions.
\[\ce{2Ag+}(aq)⟶\ce{2Ag}(s)\]
\[\ce{Cu}(s)⟶\ce{Cu^2+}(aq)\]
Step 2: Identify the anode and cathode.
The anode is the \(\ce{Cu}(s)\)since it increases in oxidation state from 0 to +2.
The cathode is the \(\ce{Ag+}(aq)\) as it decreases in oxidation state from +1 to 0.
Step 3: Construct the Cell Diagram.
\[\ce{Cu}(s)│\ce{Cu^2+}(aq)║\ce{Ag+}(aq)│\ce{Ag}(s)\]
Q3. \[\ce{Mn}(s)+\ce{Sn(NO3)2}(aq)⟶\ce{Mn(NO3)2}(aq)+\ce{Sn}(s)\]
Step 1: Write the two half-reactions.
\[\ce{Mn}(s)⟶\ce{Mn(NO3)2}(aq)\]
\[\ce{Sn(NO3)2}(aq)⟶\ce{Mn(NO3)2}(aq)\]
Step 2: Identify the anode and cathode.
The anode is the \(\ce{Mn}(s)\) since it increases in oxidation state from 0 to +2.
The cathode is the \(\ce{Sn(NO3)2}(aq)\) as it decreases in oxidation state from +2 to 0.
Step 3: Construct the Cell Diagram.
\[\ce{Mn}(s)│\ce{Mn^2+}(aq)║\ce{Sn^2+}(aq)│\ce{Sn}(s)\]
Q4. \[\ce{3Cu(NO3)}(aq)+\ce{Au(NO3)3}(aq)⟶\ce{3Cu(NO3)2}(aq)+\ce{Au}(s)\]
Step 1: Write the two half-reactions.
\[\ce{3CuNO3}(aq)⟶\ce{3Cu(NO3)2}(aq)\]
\[\ce{Au(NO3)3}(aq)⟶\ce{Au}(s)\]
Step 2: Identify the anode and cathode.
The anode is the \(\ce{3CuNO3}(aq)\) since it increases in oxidation state from +1 to +2.
The cathode is the \(\ce{Au(NO3)3}(aq)\) as it decreases in oxidation state from +3 to 0.
Step 3: Construct the Cell Diagram. Because no solid exists on the anode side we must add platinum as an inert electrode.
\[\ce{Pt}(s)│\ce{Cu+}(aq),\: \ce{Cu^2+}(aq)║\ce{Au^3+}(aq)│\ce{Au}(s)\]
Q19.1.10
Would you expect an aqueous manganese(VII) oxide solution to have a pH greater or less than 7.0? Justify your answer.
S19.1.10
In order to solve this problem it is important to be knowledgeable about oxidation states. Manganese(VII) oxide consists of two elements, Manganese (Mn) and Oxygen (O). From the (VII) we can determine that Manganese has an oxidation state of +7. Because the complex is neutral the overall oxidation state must be zero. Because Oxygen has an oxidation state of -2 (and because there are two Manganese atoms) it will take 7 Oxygens to equalize the charges. The chemical formula for this is \(\ce{Mn_{2}O_{7}}(aq)\). In solution this will dissociate and in relation to the Lewis acid-base theory, the Lewis acid accepts lone pair electrons; thus, it is also known as the electron pair acceptor. This may be any chemical species. Acids are substances that must be lower than 7. Therefore, oxides of manganese is most likely going to become more acidic in (aq) solutions if the oxidation number increases.
19.3: Spectroscopic and Magnetic Properties of Coordination Compounds
Q19.3.2
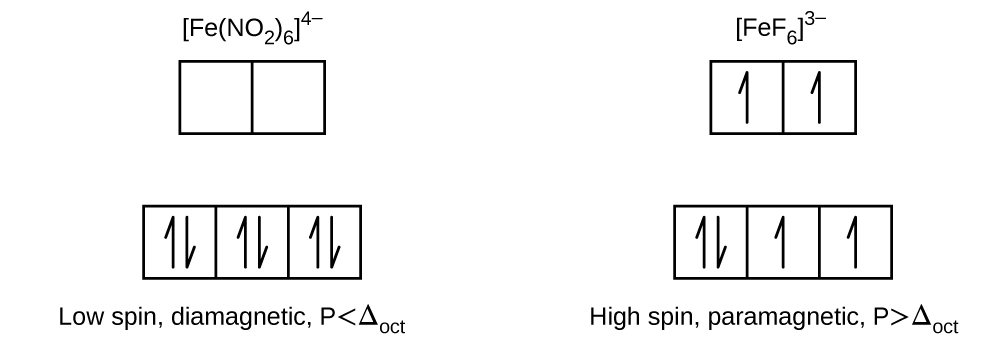
Draw the crystal field diagrams for \([{Fe(NO_{2})_{6}}]^{4-}\) and \([{FeF_{6}}]^{3-}\). State whether each complex is high spin or low spin, paramagnetic or diamagnetic, and compare \(\Delta _{oct}\) to \(P\) for each complex.
S19.3.2
In order to solve these problems we must first be knowledgeable of crystal field theory (CFT). CFT allows us to understand, interpret, and predict the colors, magnetic behavior, and some structures of coordination compounds of transition metals. Because both of these complexes have six ligands they are octahedral complexes, meaning their orbitals are split into two sublevels, the higher level is called \(e_{g}\) (which consists of the hybridized \(d_{z^2}\) and \(d_{x^2−y^2}\) orbitals) and the lower level is the \(t_{2g}\) (consisting of the \(d_{xy}\), \(d_{xz}\), and \(d_{yz}\) orbitals). Next it is important to recognize that difference in energy between the two levels is called the crystal field splitting otherwise known as \(\Delta _{oct}\). \(\Delta _{oct}\) is determined by several factors, namely the strength of the aligned bound to the metal atom, a sample series is shown below. In addition, electron pairing energy or \(P\) is the amount of energy required to pair electrons, if this is less than the crystal field splitting than electrons will stay in the bottom level and the converse is true, if \(\Delta _{oct}\) is less than \(P\) than electrons will inhabit both levels before pairing. The existence of unpaired elections determines the magnetism (and spin) of the species, if there are any unpaired electrons the species is paramagnetic (and high spin). In order to be diamagnetic (and low spin) there must be no unpaired electrons.
\[\large \underset{\textrm{The are a few ligands of the spectrochemical series, in order of increasing field strength of the ligand}}{\xrightarrow{\ce{I- <Br- <Cl- <F- <H2O<C2O4^2- <NH3<\mathit{en}<NO2- <CN-}}}\]
To solve these problems we will use a four step process:
Step 1: Determine the number of electrons.
Step 2: Determine strength of ligand.
Step 3: Determine spin and magnetism
Step 4: Draw CTF diagram
Q1. \([{Fe(NO_{2})_{6}}]^{4-}\)
Step 1: Determine the number of electrons.
Because \(NO_{2}\) carries a -1 charge and the overall complex has a charge of -4 we can determine that the Iron is Iron (II) and that there are six electrons.
Step 2: Determine strength of ligand.
In this case the ligand is \(NO_{2}\) which is a rather strong ligand, leading to a large \(\Delta _{oct}\) (much larger than \(P\)). This leads to the electrons occupying the \(t_{2g}\) orbitals first.
Step 3: Determine spin and magnetism
There are six electrons that occupy the three lower orbitals in pairs, therefor there are no unpaired electrons so the atom is low spin and diamagnetic.
Step 4: Draw CTF diagram
See Below
Q2. \([{FeF_{6}}]^{3-}\)
Step 1: Determine the number of electrons.
Because \(F\) carries a -1 charge and the overall complex has a charge of -3 we can determine that the Iron is Iron (III) and that there are six electrons.
Step 2: Determine strength of ligand.
In this case the ligand is \(F_{6}\) which is a rather weak ligand, leading to a small \(\Delta _{oct}\) (much smaller than \(P\)). This leads to the electrons occupying all the orbitals first as single electrons.
Step 3: Determine spin and magnetism
There are six electrons that occupy all the orbitals as single electrons first then begin to fill the lower orbitals in pairs, this leads to four unpaired electrons and because of this the atom is lower orbitals in pairs so the atom is high spin and paramagnetic.
Step 4: Draw CTF diagram
See Below
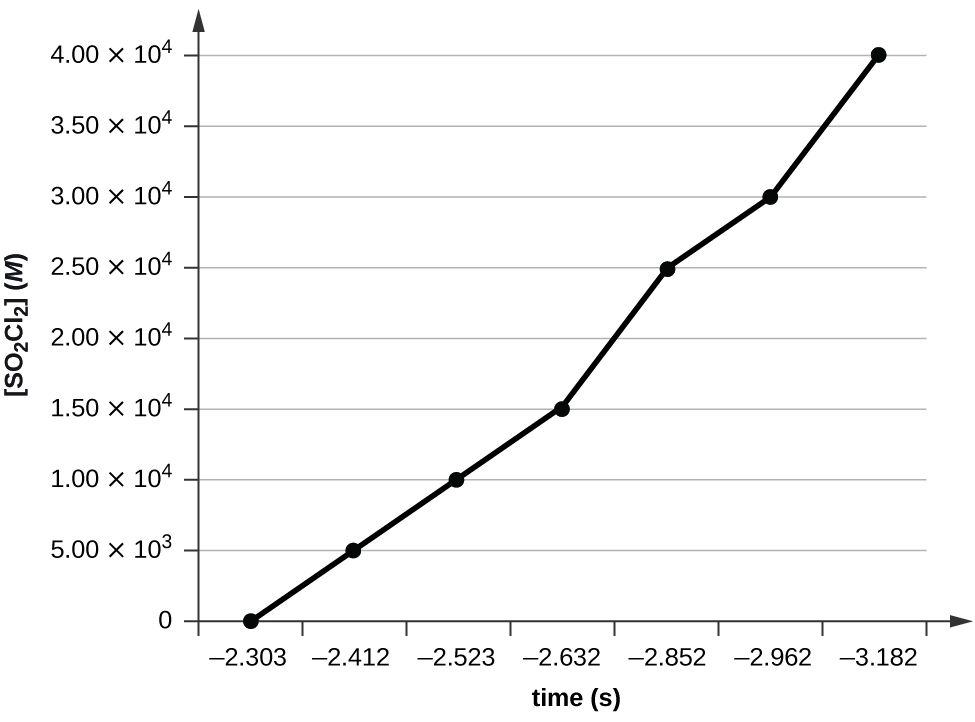
12.4: Integrated Rate Laws
Q12.4.2
Use the data provided to graphically determine the order and rate constant of the following reaction: \(\ce{SO2Cl2 ⟶ SO2 + Cl2}\)
| Time (s) | 0 | 5.00 × 103 | 1.00 × 104 | 1.50 × 104 | 2.50 × 104 | 3.00 × 104 | 4.00 × 104 |
|---|---|---|---|---|---|---|---|
| [SO2Cl2] (M) | 0.100 | 0.0896 | 0.0802 | 0.0719 | 0.0577 | 0.0517 | 0.0415 |
Plotting a graph of \(\ce{ln}[SO_{2}Cl_{2}]\) versus t reveals a linear trend; therefore we know this is a first-order reaction:
S12.4.2
This problem is solved by utilizing our knowledge of the fact that this is a first order reaction, we can therefore use the first order rate law relating the rate constant \(k\)
to the initial concentration \([A]_{0}\) and the concentration \([A]_{t}\) present after any given time \(t\) can be derived for a first-order reaction and shown to be:
\[\ln\left(\dfrac{[A]_t}{[A]_0}\right)=kt\]
For this problem we will take \([A]_{0}\) to equal 0.100M and \([A]_{t}\) to equal 0.0415M after \(t\) equals 4.00 × 104 seconds.
All that is left to do is plug in the values and solve:
\[\ln\left(\dfrac{[A]_t}{[A]_0}\right)=kt\]
\[ln\left ( \frac{0.0415M}{0.100M} \right )=k(4.00\times 10^{4}s)\]
\[ln(.415)=k(4.00\times 10^{4}s)\]
\[-.8794767588=k(4.00\times 10^{4}s)\]
\[-2.1986919\times 10^{5}=k\]
Because this is a first order reaction we know that the units for the rate constant must be \(s^{-1}\) and so
\[k=-2.20\times 10^5s^{-1}\]
Q12.7.5
For each of the following pairs of reaction diagrams, identify which of the pairs is catalyzed:
(a)
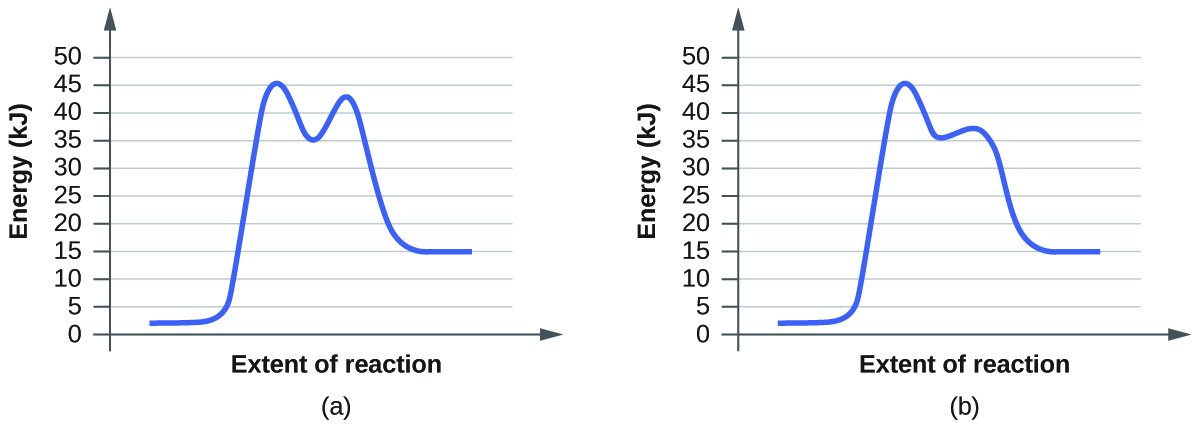
(b)
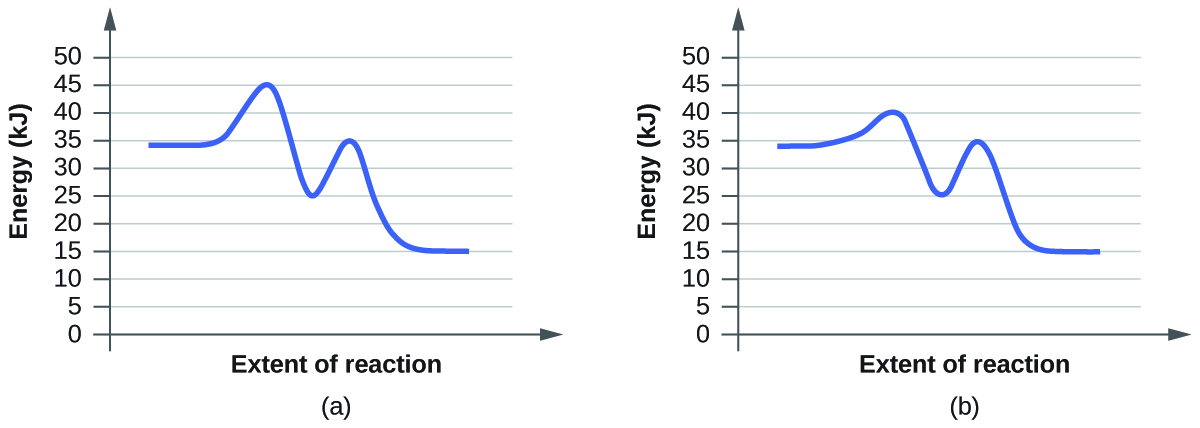
S12.7.5
When a reaction has been catalyzed the ensuing effect is a lowering of the transition energy state. This is because the catalyst speeds up the rate of a reaction by lowering the activation energy. Activation energy can be calculated by the equation \(E_{a}=E(transition)-E(reagent)\) with values usually being presented in kilojoules or kJ.
Q1. Graph A shows a first activation energy of \(E_{a}=45kJ-2.5kJ=42.5kJ\) and a second activation energy of \(E_{a}=42.5kJ-2.5kJ=40kJ\)
Graph B shows a first activation energy of \(E_{a}=45kJ-2.5kJ=42.5kJ\) and a second activation energy of \(E_{a}=37.5kJ-2.5kJ=35kJ\)
Because the activation energy is lower in graph B it must be the graph of the catalyzed reaction.
Q2. Graph A shows a first activation energy of \(E_{a}=45kJ-35kJ=10kJ\) and a second activation energy of \(E_{a}=35kJ-35kJ=0kJ\)
Graph B shows a first activation energy of \(E_{a}=40kJ-35kJ=5kJ\) and a second activation energy of \(E_{a}=40kJ-35kJ=5kJ\)
Because the activation energy is lower in graph B it must be the graph of the catalyzed reaction.
21.4: Radioactive Decay
Q21.4.28
Write a balanced equation for each of the following nuclear reactions:
- mercury-180 decays into platinum-176
- zirconium-90 and an electron are produced by the decay of an unstable nucleus
- thorium-232 decays and produces an alpha particle and a radium-228 nucleus, which decays into actinium-228 by beta decay
- neon-19 decays into fluorine-19
S21.4.28
In order to solve these problems we must be familiar with notation the different types of radioactive decay. It is also important to realize the changes in the number of protons and other particles.
| Identity | Symbol | Charge | Mass (amu) |
|---|---|---|---|
| helium nucleus | \(^4_2\alpha\) | +2 | 4.001506 |
| electron | \(^0_{-1}\beta\) or \(\beta ^-\) | −1 | 0.000549 |
| photon | \(_0^0\gamma\) | — | — |
| neutron | \(^1_0\textrm n\) | 0 | 1.008665 |
| proton | \(^1_1\textrm p\) | +1 | 1.007276 |
| positron | \(^0_{+1}\beta\) or \(\beta ^+\) |
+1 | 0.000549 |
Write a balanced equation for each of the following nuclear reactions:
Q1. mercury-180 decays into platinum-176
In this reaction mercury-180 loses an alpha particle, the equivalent of a helium atom and platinum-176.
\[_{80}^{180}\textrm{Hg}\rightarrow _{176}^{78}\textrm{Pt}+^4_2\alpha\]
Q2. zirconium-90 and an electron are produced by the decay of an unstable nucleus
In this reaction zirconium-90 and an electron are produced by the decay of an unstable atom, through addition it can be determined that the origional nucleus was yttrium-90.
\[_{39}^{90}\textrm{Y}\rightarrow _{40}^{90}\textrm{Zr}+^0_{-1}\beta\]
Q3. thorium-232 decays and produces an alpha particle and a radium-228 nucleus, which decays into actinium-228 by beta decay
In the first reaction thorium-232 loses an alpha particle, the equivalent of a helium atom and then in the second reaction radium-228 gains a proton through beta decay, creating actinium-228 and and electron.
Reaction 1: \[_{90}^{232}\textrm{Th}\rightarrow _{88}^{228}\textrm{R}+^4_2\alpha\]
Reaction 2: \[_{88}^{228}\textrm{Ra}\rightarrow _{89}^{228}\textrm{Ac}+^0_{-1}\beta\]
Q4. neon-19 decays into fluorine-19
In this reaction neon-19 decays and produces fluorine-19 and a beta particle.
\[_{10}^{19}\textrm{Ne}\rightarrow _{9}^{19}\textrm{F}+^0_{+1}\beta\]
20.3: Voltaic Cells
Q20.3.14
For each redox reaction, write the half-reactions and draw the cell diagram for a galvanic cell in which the overall reaction occurs spontaneously. Identify each electrode as either positive or negative.
1. \(\ce{Ag}(s)+\ce{Fe^3+}(aq)⟶\ce{Ag^+}(aq)+\ce{Fe^2+}(aq)\)
2. \(\ce{Fe^3+}(aq)+\ce{1/2H2}(g)⟶\ce{Fe^2+}(aq)+\ce{H^+}(aq)\)
S20.3.14
For these problems it is important to keep in mind that you must determine the proper reactants and products at both the anode nd the cathode and keep track of the states of these elements. In addition, it is necessary to be familiar with electrochemical cell notation and terms:
- Cellular notation is read from left to right
- The left denotes the anode and the right the cathode (these are referred to as half-cells)
- Anode: The anode is where the oxidation reaction takes place. In other words, this is where the metal loses electrons.
- Cathode: The cathode is where the reduction reaction takes place. This is where the metal electrode gains electrons.
- | separates different phases (a comma is used to differentiate between species of the same phase)
- || is used to differentiate between the anode and cathode, representing the salt bridge
- Concentrations are often written in the parentheses after the phase notation (the lack of concentrations denotes standard conditions, ex. 1M)
When solving these problems we will use a three step process:
Step 1: Write the two half-reactions.
Step 2: Identify the cathode and anode.
Step 3: Construct the Cell Diagram.
Q1. \[\ce{Ag}(s)+\ce{Fe^3+}(aq)⟶\ce{Ag^+}(aq)+\ce{Fe^2+}(aq)\]
Step 1: Write the two half-reactions.
\[\ce{Ag}(s)⟶\ce{Ag^+}(aq)\]
\[\ce{Fe^3+}(aq)⟶\ce{Fe^2+}(aq)\]
Step 2: Identify the anode and cathode.
The anode is the \(\ce{Ag}(s)\) since it increases in oxidation state from 0 to +1. This is the negative electrode.
The cathode is the \(\ce{Fe^3+}(aq)\) as it decreases in oxidation state from +3 to +2. This is the positive electrode.
Step 3: Construct the Cell Diagram. Because no solid exists on the cathode side we must add platinum as an inert electrode.
\[\ce{Ag}(s)│\ce{Ag^+}(aq)║\ce{Fe^3+}(aq),\: \ce{Fe^2+}(aq)│\ce{Pt}(s)\]
Q2. \[\ce{Fe^3+}(aq)+\ce{1/2H2}(g)⟶\ce{Fe^2+}(aq)+\ce{H^+}(aq)\]
Step 1: Write the two half-reactions.
\[\ce{Fe^3+}(s)⟶\ce{Fe^2+}(aq)\]
\[\ce{1/2H2}(g)⟶\ce{H^+}(aq)\]
Step 2: Identify the anode and cathode.
The anode is the \(\ce{1/2H2}(g)\) since it increases in oxidation state from 0 to +1. This is the negative electrode.
The cathode is the \(\ce{Fe^3+}(aq)\) as it decreases in oxidation state from +3 to +2. This is the positive electrode.
Step 3: Construct the Cell Diagram. Because no solid exists on the anode or cathode side we must add platinum as an inert electrode.
\[\ce{Pt}(s)│\ce{1/2H2}(g),\: \ce{H^+}(aq)║\ce{Fe^3+}(aq),\: \ce{Fe^2+}(aq)|\ce{Pt}(s)\]
20.5: Free Energy and Redox Reactions
Q20.5.27
Under acidic conditions, ideally any half-reaction with E° > 1.23 V will oxidize water via the reaction \(\ce{O2}(g)+\ce{4H^+}(aq)+\ce{4e^-}⟶\ce{2H2O}(l)\).
- Will aqueous acidic \(\ce{KMnO}_{4}\) evolve oxygen with the formation of \(\ce{MnO}_{2}\) ?
- At pH 14.00, what is E° for the oxidation of water by aqueous \(\ce{KMnO}_{4}\) (1 M) with the formation of \(\ce{MnO}_{2}\)?
- At pH 14.00, will water be oxidized if you are trying to form \(\ce{MnO}_{2}\) from \(\ce{2MnO}{_{4}}^{2-}\) via the reaction \(\ce{2MnO}{_{4}}^{2-}(aq)+\ce{2H_{2}O}(l)⟶\ce{2MnO_{2}}(s)+\ce{O}_{2}(g)+\ce{4OH^-}(aq)\)?
S20.5.27
In order to solve these problems we need access to a list of Standard Reduction Potentials (SRPs) such as Table P2. From there you can acquire the values and equations needed to evaluate the situations.
Q1. Will aqueous acidic \(\ce{KMnO}_{4}\) evolve oxygen with the formation of \(\ce{MnO}_{2}\)
From the table of SRPs you can see that the \(\ce{KMnO}_{4}\) equation is above the \(H_{2}O\). From that we can determine that the \(\ce{KMnO}_{4}\) oxidizes the water. Because \(\ce{KMnO}_{4}\) oxidizes it is the anode and the water is therefore the cathode.
Next we are to use the equation \(E^{o}=E_{cathode}^{o}-E_{anode}^{o}\) to find \(E^{o}\)
\[E^{o}=E_{cathode}^{o}-E_{anode}^{o}\]
\[E^{o}=1.68 V-1.23V\]
\[E^{o}=0.45V\]
Yes, aqueous acidic \(\ce{KMnO}_{4}\) will evolve oxygen with the formation of \(\ce{MnO}_{2}\).
Q2. At pH 14.00, what is \(E^{o}\) for the oxidation of water by aqueous \(\ce{KMnO}_{4}\) (1 M) with the formation of \(\ce{MnO}_{2}\)?
From the table of SRPs you can see that the \(\ce{KMnO}_{4}\) equation is above the \(H_{2}O\). From that we can determine that the \(\ce{KMnO}_{4}\) oxidizes the water. Because \(\ce{KMnO}_{4}\) oxidizes it is the anode and the water is therefore the cathode.
Next we are to use the equation \(E^{o}=E_{cathode}^{o}-E_{anode}^{o}\) to find \(E^{o}\)
\[E^{o}=E_{cathode}^{o}-E_{anode}^{o}\]
\[E^{o}=1.424 V-1.23V\]
\[E^{o}=0.194V\]
The \(E^{o}\) for the oxidation of water by aqueous \(\ce{KMnO}_{4}\) (1 M) with the formation of \(\ce{MnO}_{2}\) is 0.194 V.
Q3. At pH 14.00, will water be oxidized if you are trying to form \(\ce{MnO}_{2}\) from \(\ce{2MnO}{_{4}}^{2-}\) via the reaction \(\ce{2MnO}{_{4}}^{2-}(aq)+\ce{2H_{2}O}(l)⟶\ce{2MnO_{2}}(s)+\ce{O}_{2}(g)+\ce{4OH^-}(aq)\)?
From the table of SRPs you can see that the \(\ce{KMnO}_{4}\) equation is above the \(H_{2}O\). From that we can determine that the \(\ce{KMnO}_{4}\) oxidizes the water. Because \(\ce{KMnO}_{4}\) oxidizes it is the anode and the water is therefore the cathode.
Next we are to use the equation \(E^{o}=E_{cathode}^{o}-E_{anode}^{o}\) to find \(E^{o}\)
\[E^{o}=E_{cathode}^{o}-E_{anode}^{o}\]
\[E^{o}=1.43 V-1.23V\]
\[E^{o}=0.20V\]
Yes, water be oxidized if you are trying to form \(\ce{MnO}_{2}\) from \(\ce{2MnO}{_{4}}^{2-}\) via the reaction \(\ce{2MnO}{_{4}}^{2-}(aq)+\ce{2H_{2}O}(l)⟶\ce{2MnO_{2}}(s)+\ce{O}_{2}(g)+\ce{4OH^-}(aq)\) and the \(E^{o}\) would be 0.20V.