Extra Credit 8
- Page ID
- 83450
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Edited by Tanya Joumblat in blue.
Q19.2A
A Lithium-ion battery has a theoretical E∘cell of 1.5 V. Find the E∘ for the reduction half-reaction:
\(\mathrm{Li^+(aq) + e^- \rightarrow Li(s)}\)
S19.2A
The E∘ for the reaction \(\mathrm{Li^+(aq) + e^- \rightarrow Li(s)}\)
equals -3.040 according to the Standard Electrode Potential Table. This value indicates the individual half-reaction's standard reduction potential in comparison to the half-reaction of Hydrogen that has an \(\ce{E^{\circ}}\) value of 0.00 V.
Q19.35B
Find \(\ce{[H2O2(aq)]}\) in cell if the voltaic cell's Ecell=1.500V.
\(\mathrm{Zn(s)|Zn^{2+}(aq) (1.00\:M) || H_2O_2(aq)(x M) | H_2O(l)}\)
Hint: use Nernst's Equation.
S19.35B
1. Find Ecell
The anode undergoes oxidation: lose of electrons
Anode: \(\mathrm{Zn(s) \rightarrow Zn^{2+}(aq) + 2e^- \hspace{68 pt}\quad E^\circ_{cell} = -0.763\:V}\)
The cathode undergoes reduction: gain of electrons
Cathode: \(\mathrm{H_2O_2(aq) + 2H^+(aq) + 2e^- \rightarrow 2H_2O(l) \quad E^\circ_{cell} = +1.763\:V}\)
Use this equation to find Ecell:
\(\displaystyle E_0cell=E_{0cathode}-E_{0anode}\)
E∘cell=+1.763V−(−0.763V)=2.526V
2. Plug into the Nernst's equation
n= number of electrons (look under your anode and cathode equations)
Find Q by following:
\(\displaystyle aA +bB \rightleftharpoons gG +hH \)
This balanced equation can be found by combining your anode and cathode half-reactions. These two half-reactions can only be combined if they have the same amount of electrons; if they don't, then one or both of your half-reaction equations would need to be multiplied by a stoichiometric coefficient in order to make them equal. In this way, when the two half-reactions are combined, the electrons can cancel out.
\(\displaystyle K_c = \dfrac{[G]^g [H]^h}{[A]^a [B]^b} \)
Plug in: But don't include solids or \(\displaystyle H_2O \) (or any liquids, really)
\(\mathrm{E = E^\circ_{cell} - \dfrac{0.0592}{n} \log \dfrac{[Zn^{2+}]}{[H_2O_2]}}\)
\(\mathrm{1.5V = 2.526V - \dfrac{0.0592}{2} \log \dfrac{[1]}{[x]}}\)
\(\displaystyle34.66=log(\frac{1}{x}) \)
use base 10 to "undo" the log
\(\displaystyle10^{34.66}=\frac{1}{x} \)
Q20.21B
Write the possible half equations to represent each of the following
- Cu2+ as an oxidizing agent
- Mn2+ as a reducing agent
S20.21B
Oxidizing agent means Cu2+ gets reduced
RIG= reduction gains electrons
The charge of Cu should decrease as electrons are added
- Cu2+ + 2e- --> Cu
Reducing agent means Mn2+ gets oxidized
OIL= oxidation loses electrons
The charge of Mn should increase as electrons are lost
2. Mn2+ --> Mn4+ + 2e-
In each half reaction, a certain number of electrons are necessary in order to balance the charges since the atoms are either gaining or losing electrons.
Q21.27B
Which of the following would you expect to have the largest overall \(\mathrm{K_f}\) and why? \(\ce{[Co(NH3)6]^3+}\), \(\ce{[Co(en)3]^3+}\), \(\ce{[Co(H2O)6]^3+}\)
S21.27B
In order to determine Kf, we need to compare the ligands in each compound; \(\displaystyle NH_3\), \(\displaystyle en\), and \(\displaystyle H_2O\). \(\displaystyle NH_3\) is a monodente ligand. \(\displaystyle en\) is a bidente ligand. \(\displaystyle H_2O\) is a monodente ligand also. Out of the three different ligands we expect the (en) ligand to have the largest overall Kf value due to the chelation effect. Experimentally, it is observed that metal complexes of polydentate/bidente ligands are significantly more stable than complexes of chemically similar monodentate ligands. The formation of a complex with bidentate groups is favored over the formation of a complex with monodentate groups because for bidentate groups, less favorable collisions between the metal ion and the ligand molecule are required. The formation constant can also be called a stability constant. So, more stable complexes will have larger formation constants.
Q24.37A
For a certain decomposition reaction, the following observations have been made:
at t=0s, [Reactant]=1.43M;
at t=44s, [Reactant]=1.21M;
at t=148s, [Reactant]=0.69M;
at t=264s, [Reactant]=0.11M.
Determine the order and half-life of this reaction.
S24.37A
To determine the reaction order: plot [Reactants] vs. Time, ln[Reactants] vs. Time, \(\mathrm{\frac{1}{[Reactants]}}\) vs. Time
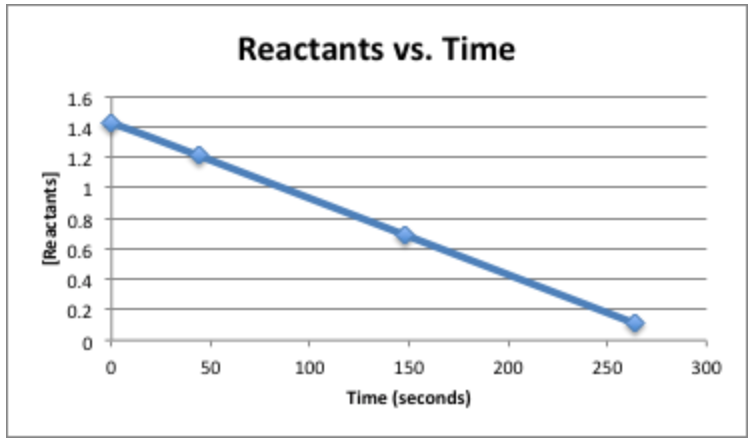
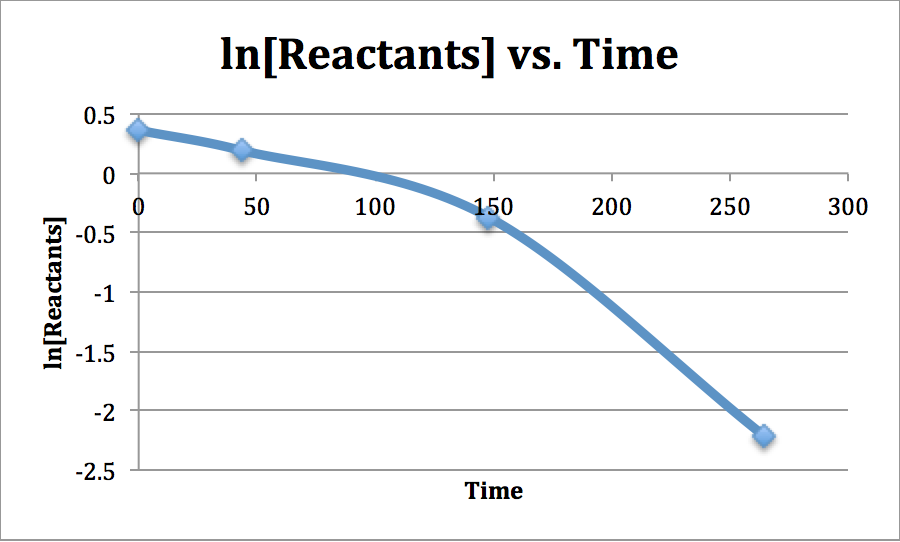
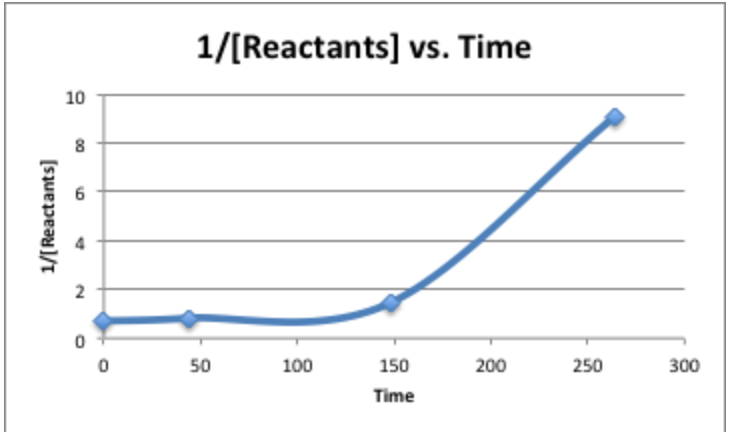
The y-axes if each plot corresponds to the integrated rate law of that particular order. For instance, the first order rate law is \(\mathrm{ln[A]_{t}=-kt+ln[A]_{0}}\). Whene compared to the y-intercept equation, y= mx+b, y=\(\ce{ln[A]_{t}}\), m=-k, x=t, and \(\ce{b=ln[A]_{0}}\).
If the plot of [Reactants] vs. Time is straight, then it is zero order.
If the plot of ln[Reactants] vs. Time is straight, then it is first order.
If the plot of \(\mathrm{\frac{1}{[Reactants]}}\) vs. Time is straight, then it is second order.
So, this reaction is of zero-order.
Determine the Half-Life:
The rate constant, k, is given by the slope of this straight line.
The formula for calculating slope is:
\(\mathrm{\frac{Y_2-Y_1}{X_2-X_1}}\)
Choose two points to use.
k= \(\mathrm{\frac{0.69M−1.43M}{148s−0s} }\)
k=−0.00500M/s.
The Zero order equation for half life is:
\(\mathrm{t_{\frac{1}{2}}=\frac{[A]_0}{2k}}\)
Plug in your initial value \(\mathrm{[A_0]}\)from the original experiment and your slope value of K and solve.
\(\mathrm{t_{\frac{1}{2}}=\frac{1.43}{2(-0.00500)}}\)
\(\mathrm{t_{\frac{1}{2}}}\)=143s
Q25.13D
Write out a nuclear equation that show the formation of an isotope element 97 with mass number 255 by the bombardment of bismuth-219 by nickel-64 nuclei followed by a succession of \(\displaystyle\alpha \) particle emission, hence find number of \(\displaystyle\alpha \) particle emission.
S25.13D
Check out Figure 5.3.1 for different kinds of nuclear decay reactions and equations.
Use this equation for alpha particle emission
\(\mathrm{_{Z}^{A}{X} \rightarrow _{A-4}^{Z-2}X^{'}\ +_{2}^{4}\alpha}\)
\(\mathrm{\ce{^{219}_{83}Bi + ^{64}_{28}Ni \rightarrow ^{255}_{97}Bk + ?}}\)
Total mass should equal 219+64=286 on each side of the equation
In general, the sum of the atomic masses on one side of the equation should be equal to the sum of the masses on the other side of the equation. This same rule applies to alpha particles. In order to ensure that you have the correct number of alpha particles you can substitute \(\ce{\alpha}\) with \(\mathrm{_{2}^{4}\textrm{He}}\).
so 286-255=28 mass emmited through alpha particles. Each alpha particle has a mass of 4.
So 28/4= 7 alpha particles
\(\mathrm{\ce{^{219}_{83}Bi + ^{64}_{28}Ni \rightarrow ^{255}_{97}Bk + 7\alpha}}\)
Q19.2
The isotope \(\mathrm{ _{55}^{137}\textrm{Cs}}\)undergoes beta emission with a half-life of 30 years.
- Write a balanced nuclear equation for this reaction.
- What fraction of Cs-137 remains in a sample of the isotope after 60 years?
- What mass of Cs will be left in a 24.0 g sample of \(\mathrm{ _{55}^{137}\textrm{Cs}}\)after 90 years?
-
What fraction of Cs-137 has decayed after 120 years?
S19.2
1. Write a balanced nuclear equation for this reaction.
\(\mathrm{_{55}^{137}\textrm{Cs} \rightarrow _{-1}^{0}\beta + {_{56}^{137}\textrm{Ba}}}\)
Check out Nuclear Decay Emission Table 5.3.1
2. What fraction of Cs-137 remains in a sample of the isotope after 60 years?
Radio active Decay follows 1st order kinetics.
Use this Equation:
\(\mathrm{ fractionremaining=0.5^{\frac{t}{t_{\frac{1}{2}}}}
}\)
\(\mathrm{fractionremaining=0.5^{_\frac{60}{30}}}\)
1/4 of the original Cs-137 remains after 60 years
3. What mass of Cs will be left in a 24.0 g sample of \(\mathrm{ _{55}^{137}\textrm{Cs}
}\) after 90 years?
\(\mathrm{ fractionremaining=0.5^{\frac{t}{t_{\frac{1}{2}}}}
}\)
\(\mathrm{fractionremaining=0.5^{_\frac{90}{30}}}\)
1/8 remains after 90 years
Multiply the fraction of Cs that is remaining by the original amount in grams in order to find the number of grams remaining after 90 years.
\(\mathrm{24\times \frac{1}{8}=3 }\)
3.0 grams of Cs-137 remains after 90 years
4. What fraction of Cs-137 has decayed after 120 years?
Again:
\(\mathrm{ fractionremaining=0.5^{\frac{t}{t_{\frac{1}{2}}}}
}\)
\(\mathrm{fractionremaining=0.5^{_\frac{120}{30}}}\)
1/16th remains
The question is asking what fraction of Cs-137 has decayed. As a result you need to subtract the fraction that remains from 1 in order to find the fraction that decayed after 120 years.
so, \(\mathrm{1-\frac{1}{16}=\frac{15}{16}}\) has decayed after 120 years
Q21.3.1
Why do scientists believe that hydrogen is the building block of all other elements? Why do scientists believe that helium-4 is the building block of the heavier elements?
S21.3.1
Hydrogen is the simplest and most abundant element there is. It contains just one proton and one electron. Fusion of hydrogen atoms produced all other elements making it a building block. Helium also has a very simple structure and is the second most common element. H-1 and He-2 combined account for 99% of all the atoms in the known universe. Many fusion reactions of helium nuclei at higher temperatures create elements with even numbers of protons and neutrons up to magnesium and then up to calcium. Eventually, the elements up to iron and nickel are formed by exchange processes at even higher temperatures

