Extra Credit 43
- Page ID
- 83440
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)19.22A
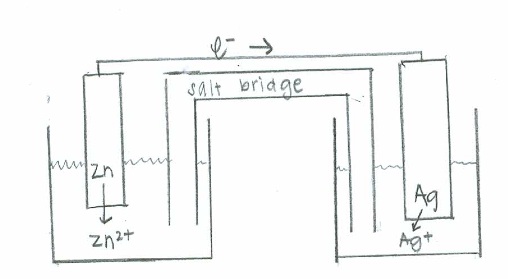
For each of the following reactions below, draw a voltaic cell. In your drawing include the anode, cathode, and show the flow of electrons. Balance the equation and calculate the E∘cell.
- Zn(s)+Ag+(aq)→Ag(s)+Zn2+(aq)
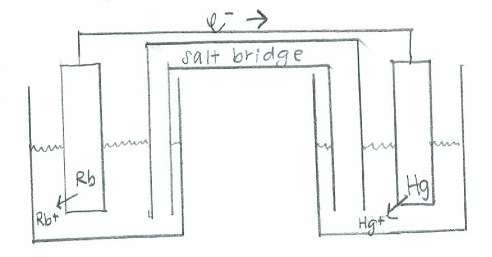
- Rb(s)+Hg2+(aq)→Rb+(aq)+Hg(s)
- F2(g)+H2O(l)→4F−(aq)+O2(g)+H+(aq)
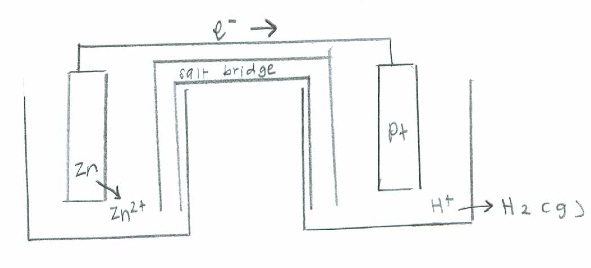
- Zn(s)+H+(aq)→Zn2+(aq)+H2(g)
Solution for 19.22A
These problems are all very similar and cover the same concepts so here is an explanation of how to approach these problems step by step and I will also go through the problem (a) step by step.
Step 1: Determine which species is being reduced and which is being oxidized.
This is done by determining the change in oxidation states of each of the species. If the species DECREASES in oxidation state, then it is REDUCED. If the species INCREASES in oxidation state, then the it is OXIDIZED.
Step 2: Balance each of the half reactions.
Oxidation: Put the species being oxidized in a reaction and add electrons so the charge is balanced for the given half reaction. (Electron(s) go on product side) Also find the reduction potential for this species and switch the sign of the potential (typically it is negative.)
Reduction: Put the species being reduced in a reaction and add electrons so the charge is balanced for the given half reaction. (Electron(s) go on the reactant side) Also find the reduction potential for this species (typically it is positive)
Step 3: Sum the equations.
Check to see if the electrons cancel out when the reactions are summed. If not, multiply a reaction or possibly both reactions by a factor so the number of electrons are equal and cancel out when the equations are summed. DO NOT change E cell of either when doing this step and make sure NO electrons are in the net reaction.
Also add together the two reduction potentials to determine the E cell of the net reaction.
Step 4: Drawing the voltaic cell.
Have two beakers filled with solutions and add metal rods to the solution and wire connecting the two rods(label one anode and one cathode) out of the water and a salt bridge between the two solutions. After this drawing is completed here is the information that is crucial for a successful drawing.
Reduction occurs at the cathode
Oxidation occurs at the anode
Electron flow is from the anode to the cathode
Using the equation \(E^\circ_{cell}= E^\circ_{cathode} - E^\circ_{anode}\), we can find the E cell of the net reaction.
Oxidation: \(\mathrm{Zn(s) \rightarrow Zn^{2+} (aq) + 2e^- \hspace{38.5 pt}\quad {E}^\circ=0.763\:V}\)
This oxidation reaction is determined by seeing that zinc is oxidized in the overall redox reaction because zinc increases in oxidation state, from 0 to 2+. Make the negative reduction potential (-0.763 V) positive 0.763 V.
Reduction: \(\mathrm{(e^- + Ag^+(aq) \rightarrow Ag (s))\times2 \hspace{24 pt}\quad E^\circ=0.337\:V}\)
This reduction reaction is determined by seeing that silver is reduced in the overall redox reaction because silver decreases in oxidation state, from +1 to 0.
Net: \(\mathrm{Zn (s) + 2Ag^+ (aq) \rightarrow 2Ag(s) + Zn^{2+}(aq) \quad E^\circ_{cell}=1.100\:V}\)
The net reaction is determined by summing together the oxidation and the reduction reaction. This is completed by first mulitplying the reduction reaction by a factor of 2 so the electrons in the two half reactions cancel out. The two equations can then be summed and make sure there are NO electrons in the net reaction. Add the two half reaction potentials together to get an E cell of 1.100 V
E
- Oxidation: \(\mathrm{(Rb(s) \rightarrow Rb^+(aq) + e^-)\times 2 \hspace{22 pt}\quad {E}^\circ=+2.93\:V}\)
Reduction: \(\mathrm{Hg^{2+}(aq) + 2e^- \rightarrow Hg (s) \hspace{43 pt}\quad E^\circ=0.86\:V}\)
Net: \(\mathrm{2Rb(s) + Hg^{2+}(aq)\rightarrow 2Rb^+(aq) + Hg (s) \quad E^\circ_{cell}=3.79\:V}\)
- Oxidation: \(\mathrm{2H_2O (l) \rightarrow O_2(g) + 4H^+(aq) + 4e^- \hspace{31 pt} \quad {E}^\circ=-1.229\:V}\)
Reduction: \(\mathrm{(2e^-+F_2(g) \rightarrow 2F^-(aq))\times2 \hspace{60 pt} \quad E^\circ=2.866\:V}\)
Net: \(\mathrm{2F_2(g) + 2H_2O(l) \rightarrow 4F^-(aq) + O_2 (g) + 4H^+ (aq) \quad E^\circ_{cell}=1.637\:V}\)

- Oxidation: \(\mathrm{Zn(s)\rightarrow Zn^{2+}(aq) + 2e^- \hspace{24 pt}\quad {E}^\circ=+0.76\:V }\)
Reduction: \(\mathrm{2H^+(aq)+ 2e^-\rightarrow H_2 (g) \hspace{26.5 pt} \quad E^\circ=0}\)
Net: \(\mathrm{Zn (s) + H^+ (aq) \rightarrow Zn^{2+} (aq) + H_2 (g) \quad E^\circ_{cell}=0.76\:V}\)
Q 20.3B
Explain the differences between the group 1 elements and transition elements based on
- oxidation states
- colors of compounds
- magnetic properties
- formation of complexes
Solution for 20.3B
For review on characteristics of group 1 elements, read "Group 1: The Alkali Metals" and for review of transition elements, go to "Transition Metals and Coordination Complexes".
- Group 1 elements all have +1 oxidation states, but the transition elements can have many different oxidation states. For example iron has two different common oxidation states, yet sodium always is +1.
- All alkali metals have their own flame colors, but transition metals form colorful compounds due to d-d electronic transitions.
- Transition metals are paramagnetic when they have unpaired electrons in the d orbital and diamagnetic when all electrons are paired. The group 1 elements are not magnetic.
- Group 1 elements form ionic compounds and transition elements form complexes compounds because of the many pairing possibilities in the d orbital.
Q 21.15A
Draw a structure for trans-dichlorobis(ethylenediamine)cobalt(III)ion. What kind of an isomer (geometric or optical) is the cis-dichlorobis(ethylenediamine)cobalt(III)ion.
Solution for 21.15A
For review on ligands and naming of complexes visit "Ligands" and "Nomenclature of Coordination Complexes" and for review on isomers, visit "Geometric Isomers"
For drawing the of trans-dichlorobis(ethylenediamine)cobalt(III)ion, you must first place the central ion, cobalt, in the center. After this step you see that is says trans-dichlor- which means that there are two chlorine atoms(dichloro) attached to the cobalt at a 180 degree angle(trans-). Then all there is left is bis(en), which means two ethylenediamine ligands are attached to the cobalt. Since ethylenediamine is bidenate ligand, the two (en) attach to the cobalt at 4 points. The charge of the ion is determined by summing the charges of each ligand; the two (en) attach to the cobalt at 4 points. The charge of the ion is determined by summing the charges of each ligands in the ion and since there is a + charge, brackets are placed around the ion with a + at the top.
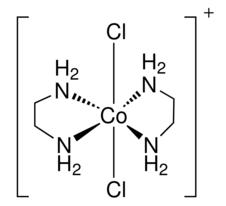
The trans-dischlorobis(ethylenediamine)cobalt(III)ion
To determine if cis-dichlorobis(ethylenediamine)cobalt(III)ion is a geometric or optical isomer, you must know the difference between the two isomers. Geometric isomers are different in terms of bonding to the central atom, while optical isomers are the same but may differ in orientation that make it appear different. In addition, optical isomers can react different to radiation based off its orientation. Since cis- orientation is a different bonding to trans-, the cis-dichlorobis(ethylenediamine)cobalt(III)ion is a geometric isomer.
Cis-dichlorobis(ethylenediamine)cobalt(III)ion is a geometric isomer.
Q 24.21B
\(\mathrm{A \rightarrow products}\) is a first order reaction. 97% of reactants decompose in 137 minutes. What is the half-life, t1/2, of this decomposition?
Solution for 24.21B
For more help see: Half-lives and Pharmacokinetics
\(\mathrm{0.03 = \dfrac{[A]_t}{[A]_0} = e^{-kt}}\)
Use the rate law for the first order reaction to determine k. 0.03 is attained by dividing 3/100 because 97% of reactants have decomposed
\(\mathrm{\ln (0.03) = \ln e^{-kt}}\)
Take the natural log of both sides to isolate k.
\(\mathrm{\ln (0.03) = - kt}\)
\(\mathrm{\ln (0.03) = - k (137\, minutes)}\)
Substitute time into the equation to find that k=0.0256
\(\mathrm{= \dfrac{\ln 2}{0.0256}}\)
Use the half life equation for first order reactions to solve for the half life by ln(2) by the value of k
t=27.08 minutes
The determined half life is 27.08 minutes
Q 25.4B
Scientists that worked on the Manhattan project made many discoveries about the nature of radioactivity. Plutonium is formed by the bombardment of Uranium-238 with neutrons, which leads to the successive emission of two beta particles in two distinct steps. What are the nuclear equations for this three-step process?
Solution for 25.4B
We know that the starting parent nuclide is Uranium-238 and that the final daughter nuclide (after a three step reaction) is Plutonium. So, the solution entails writing the equations in three distinct steps.
- \(\mathrm{\ce{^{238}_{92}U} + {^1_0n} \rightarrow \ce{^{239}_{92}U}}\)
The first step in this process is the bombardment with neutrons, so a neutron is added to the reactant side with uranium-238.
\(\mathrm{\textrm{A: } 238 + 1 = 239}\)
\(\mathrm{\textrm{Z: }92 + 0 = 92}\)
By adding a neutron, it increases the mass number by 1, but the number of protons do not change.
The resulting species is uranium-239.
- \(\mathrm{\ce{^{239}_{92}U} \rightarrow \ce{^{239}_{93}Np} + \ce{^0_{-1}\beta}}\)
The next step is a beta particle emission from the uranium-239, so the beta particle is placed on the product side.
\(\mathrm{\textrm{A: }239 = 239 + 0}\)
\(\mathrm{\textrm{Z: } 92 = 93 +(-1)}\)
The emission of a beta particle does not change the mass number, but it does change the number of protons by 1 as seen in the calculation of Z.
The resulting species is neptunium-239
- \(\mathrm{\ce{^{239}_{93}Np} \rightarrow \ce{^{239}_{94}Pu} + \ce{^0_{-1}\beta}}\)
The next step is a beta particle emission from the neptunium-239, so the beta particle is placed on the product side.
\(\mathrm{\textrm{A: }239 = 239 + 0}\)
\(\mathrm{\textrm{Z: }93 = 94 +(-1)}\)
The emission of beta particle does not change the mass number, but it does change the number of protons by 1 as seen in the calculation of Z.
The resulting species is plutonium-239
Q 25.41A
Which member of the following pairs of nuclides would you expect to be the most abundant in natural sources:
- \(\ce{^{12}_6C}\) or \(\ce{^{14}_6C}\)
- \(\ce{^{18}_8O}\) or \(\ce{^{19}_8O}\)
Explain your reasoning.
Solution for 25.41A
For review on this topic, visit the page "Decay Pathways".
a. \(\ce{^{12}_6C}\), the neutron to proton ratio is 1-to-1, (6 protons and 6 neutrons) which alludes to stability for elements with atomic number less than 20.
b. \(\ce{^{18}_8O}\), even though there is no 1-to-1, this isotope has an even number of both protons and neutrons (8 protons to 10 neutrons), which is associated with being a stable isotope.
Q 21.2.22
Using the information provided in Chapter 33, complete each reaction and calculate the amount of energy released from each in kilojoules.
- 194Tl → ? + β+
- 171Pt → ? + α
- 214Pb → ? + β−
Solution for 21.2.22
The following information will be used to find the answers for this question
Mass proton: 1.007316 amu
Mass neutron: 1.008701 amu
deltaE = deltaM * c2
c = 2.998 x 108 m/sec
a. 194Tl → 19480Hg + β+
To complete the reaction, the number of protons will decrease by 1 because a positron is emitted from thallium. Therefore it will decay to mercury, but the mass number will remain the same.
To calculate the energy released in kilojoules, you need to use the equation deltaE = deltaM * c2 where deltaM is the difference in masses.
DeltaM is calculated in amu by the number of protons and neutrons in the species and it is final minus initial
Mass final: 195.5772 amu Mass initial: 195.5758 amu deltaM = Mf - Mi = 0.0014 amu
However, since the mass units are in amu, they need to be converted to kilograms. (0.0014 amu) (\(\frac{1.6605x10^{-27}kg}{1 amu})\) = \(2.3 x 10^{-30} kg\)
Now simply plug into formula deltaE = deltaM * c2 to get energy released.
deltaE = \(2.3x10^{-30}\)(2.998x108)2
deltaE = 2.1 x 10-13 J
Convert to kilojoules by dividing by a factor of 1000 and you're done!
delta E = energy released = 2.1 x 10-16 KJ
b. 171Pt → 16776Os+ α
To complete the reaction, the number of protons will decrease by 2 and the mass number will decrease by 4 because an alpha particle is emitted from platinum. Therefore it will decay to osmium with mass number of 167.
Mf = 172.379841 amu Mi = 172.379834 amu deltaM =0.029 amu = 4.9 x 10-29 kg
deltaE = (4.9 x 10-29 kg)(2.998x108)2
deltaE = 4.4 x 10-12 J
deltaE = 4.4 x 10-15 KJ
c. 214Pb → 21483Bi + β−
To complete the reaction, the number of protons will increase by 1 because a beta particle is emitted from lead. Therefore the species will become bismuth, but the mass number will remain the same.
Mf = 215.748444 Mi = 215.747059 amu deltaM =0.0014 amu= 2.3 x 10-30 kg
deltaE = (2.3 x 10-30 kg)(2.998x108)2
deltaE = 2.07 x 10-13 J
deltaE = 2.07 x 10-16 KJ
Q 20.3.8
Consider the following spontaneous redox reaction: NO3−(aq) + H+(aq) + SO32−(aq) → SO42−(aq) + HNO2(aq).
- Write the two half-reactions for this overall reaction
- If the reaction is carried out in a galvanic cell using an inert electrode in each compartment, which electrode corresponds to which half-reaction?
- Which electrode is negatively charged, and which is positively charged?
Solution for 20.3.8
- Write the two half-reactions for this overall reaction.
reduction: 2e- + 3H+(aq)+ NO3(aq) → HNO2(aq) + H2O(l)
oxidation: H2O(l) + SO32-(aq) → SO42-(aq) + 2H+(aq) +2e-
These reactions are determined by first figuring out which reaction is reduced or oxidized
Oxygens are then balanced by adding water
Then balance hydrogens by adding H+ ions
Finally balance the charges with electrons, and these two half reactions will give the net redox reaction the question gave
b. Cathode is NO3 and Anode is SO32-. This is because NO3 is reduced, and SO32- is oxidized.
Cathode is where reduction always occurs
Anode is where oxidation always occurs
c. Cathode is positively charged while the anode is negatively charged
Cathode is always positively charged
Anode is always negatively charged

