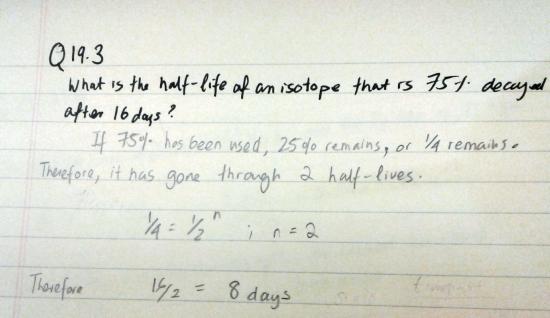Extra Credit 9
- Page ID
- 83565
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
If images are unreadable, press ctrl and + and the same time to zoom in in your web browser.
Q19.2B
Use the table of Standard Reduction Potential to predict whether these reactions will happen spontaneously.
- \(\mathrm{2Ag^+(aq) + Cu(s) \rightarrow 2Ag(s) + Cu^{2+}(aq)}\)
- \(\mathrm{Fe^{3+}(aq) + Na(s) \rightarrow Fe^{2+}(aq) + Na^+(aq)}\)
- \(\mathrm{Zn^{2+}(aq) + 2I^-(aq) \rightarrow Zn(s) + I_2(l)}\)
S19.2B
MathJax:
First break the full reaction into two half reactions:
Reduction : \(\mathrm{Ag^+(aq) +e^- \rightarrow Ag(s) }\) \(\mathrm E_ {reduction} =0.800V\)2Ag(s) }\) \(\mathrm E_ {reduction} =0.800V\)
Oxidation: \(\mathrm{Cu(s) \rightarrow 2e^- + Cu(s)}\) \(\mathrm E_ {oxidation} =-0.340V\)
The sign for the standard potential for the \(\mathrm E_ {reduction} =0.340V\) for copper has been reversed to \(\mathrm E_ {oxidation} =-0.340V\) as it is an oxidation reaction.
Hence,
\(\mathrm E_ {cell}=E_{reduction} + E_{oxidation}\)
0.800V + (-0.340 V) = 0.460V
The \(\mathrm E_ {cell}\) is positive at 0.460, making it a spontaneous reaction.
MathJax:
First break the full reaction into two half reactions in order to determine which side is being oxidized :
Reduction : \(\mathrm{Fe^{3+} + e^- \rightarrow Fe^{2+} }\) \(\mathrm E_ {reduction} =0.771V\)
Oxidation: \(\mathrm{Na \rightarrow e^- + Na^+ }\) \(\mathrm E_ {oxidation} =-2.713\)
The sign for the standard potential for the \(\mathrm E_ {reduction} =-2.713V\) for sodium has been reversed to \(\mathrm E_ {oxidation} =2.713\) as it is an oxidation reaction.
Hence,
\(\mathrm E_ {cell}=E_{reduction} + E_{oxidation}\)
0.771V + (2.713 V) = 3.484V
The \(\mathrm E_ {cell}\) is positive at 3.484V, making it a spontaneous reaction.
MathJax:
First break the full reaction into two half reactions:
Reduction : \(\mathrm{Zn^{2+} + 2e^- \rightarrow Zn^{2+} }\) \(\mathrm E_ {reduction} =-0.763V\)
Oxidation: \(\mathrm{2I^- \rightarrow 2e^- + I_2 }\) \(\mathrm E_ {oxidation} =-0.535\)
The sign for the standard potential for the \(\mathrm E_ {reduction} =0.535\) for iodine has been reversed to \(\mathrm E_ {oxidation} =-0.535\) as it is an oxidation reaction.
Hence,
\(\mathrm E_ {cell}=E_{reduction} + E_{oxidation}\)
-0.763V + (-0.535 V) = -1.298V
The \(\mathrm E_ c\) is negative at -1.298V, making it a not a spontaneous reaction.
-
In order to solve for the \(\mathrm {[Cl^-]}\) in the cell, calculate the \(\mathrm {E _{cell}}\)
-
First break the full reaction into two half reactions and since Ag is on the right side of the cell diagram it is the anode thus being oxidize and since Cl is on the left side it is the cathode side thus being reduced:
Reduction : \(\mathrm{Ag^+(aq) + e^- \rightarrow Ag(s) }\) \(\mathrm E_ {oxidation} =-0.800V\)
Oxidation: \(\mathrm{Cl_2 \rightarrow 2e^- + 2Cl^- }\) \(\mathrm E_ {reduction} = 1.358V\)
The sign for the standard potential for the \(\mathrm E_ {reduction} =0.8V\) has been reversed to \(\mathrm E_ {oxidation} =-0.8V\) as it is an oxidation reaction.
Hence,
\(\mathrm E^{∘}_ {cell}=E_{reduction} + E_{oxidation}\)
(1.340 V)+(-0.8V) =0.558 V
Using the Nernst Equation as it is non-standard conditions:
\(\mathrm E_{cell} \) = \(\mathrm E^{∘}_{cell} \) - \(\mathrm{\frac{0.0592}{n}}\) x Log Q
The number of electrons transferred (n)= 2
The concentration of \(\mathrm Ag^+\) = 0.40
The concentration of \(\mathrm Cl_2\) = 0.60
The concentration of \(\mathrm Cl^-\) is unknown
Q = \(\mathrm{\frac{concentration\, of\, products}{concentration\, of \,reactants}}\)
Plugging in the known values gives us:
\(\mathrm 0.5465 \) = \(\mathrm 0.558\) - \(\mathrm{\frac{0.0592}{2}}\) x log\(\mathrm{\frac{(0.40^2)(x^2)}{0.60}}\)
Solving for x, which is the chloride concentration, gives us: 3.03M
Q20.21C
Write an equation using \(mathrm{Mn^{2+}}\) ion as an oxidizing agent in basic solution. Write an equation using Fe(s) as a reducing agent.
\(\mathrm{Mn^2+} \) as an oxidizing agent. (Ignore the picture please he did it wrong -Max)
This would mean that \(\mathrm{Mn^2+} \) is involved in a reduction half reaction in which it gains electrons. So we would have to start with the half reaction in which\(\mathrm{Mn^2+} \) is being reduced:
MnO4- (aq) \({\rightarrow}\) Mn2+ (aq)
However this half equation is unbalanced, so we have to first balance the oxygens by adding 4 water molecules to the products side.Then we have to balance the hydrogens by adding 8 hydrogen ions to the reactants side. Finally, we have to balance the charge by adding 5 electrons to the reactants side.
MnO4- (aq) + 8H+ (aq) + 5e- \({\rightarrow}\) Mn2+ (aq) +4H2O (l)
However this balanced reaction is in an acidic solution due to hydrogen ions. In order for this reaction to be in basic solution, there must be \(\mathrm{OH^+}\) ions not hydrogen ions in order to do this we have to 8 \(\mathrm{OH^+}\) to both sides. Thus resulting in
MnO4- (aq) + 4H2O (l) + 5e- \({\rightarrow}\) Mn2+ (aq) +8\(\mathrm{OH^+}\) (aq)
This shows that Mn goes from 7+ charge to a 2+ charge as it is gaining electrons
\(\mathrm{Fe(s)} \) as a reducing agent.
This would mean that \(\mathrm{Fe(s)} \) is involved in an oxidation reaction in which is looses electrons. Hence, \(\mathrm{Fe(s)} \) will be oxidized as shown below:
\(\mathrm{Fe(s) ^- \rightarrow Fe^+ + e^- }\)
Fe goes from 0 to +1 and is being oxidized as it is losing electrons.
Q24.1A 
In the reaction 4A+3B→2C+3D reaction A is found to disappear at a rate of 5.1 X 10-5 Ms-1

B. Rate of Disappearance of B
\(\mathrm{reaction \ rate} \) x \(\mathrm{coefficient \ of \ B} \) = \(\mathrm{reaction \ rate} \)
Therefore,
\(\mathrm{1.3 x 10^-5} \) x \(\mathrm{3} \) = \(\mathrm{3.8 x 10^-5} \)
since the coefficient of B is 3.
C. Rate of formation of C =
\(\mathrm{reaction \ rate} \) x \(\mathrm{coefficient \ of \ C} \) = \(\mathrm{reaction \ rate} \)
Therefore,
\(\mathrm{1.3 x 10^-5} \) x \(\mathrm{2} \) = \(\mathrm{2.6 x 10^-5} \)
since the coefficient of C is 2
Q24.43A
Does the half-life of a reaction get longer or shorter as initial reactant concentration increases and why? Please answer for a) zero-order reactions b) second-order reactions.

The zeroth order reaction for the half reaction runs as follows:
\(\mathrm t_{1/2}\) = \(\mathrm{\frac{[reactant]}{2k}}\)
This indicates that the half life is proportional to initial reactant concentration. If, therefore, the reaction's initial reactant concentration were to increase, the reaction would go on for longer.
\(\mathrm t_{1/2}\) = \(\mathrm{\frac{1]}{k[reactant]}}\)
This indicates that the half-life is proportional inversely to the initial reactant concentration. Therefore, if the reaction's initial reactant concentration were to increase, the reaction would decrease in time.
- What is the rate of reaction?
- What is the rate of disappearance of B?
- What is the rate of formation of C?
Conclusion: The duration of half-life depends the order of the reaction.
Q25.21A
The disintegration rate for a sample containing Sr9038 as the only radioactive nuclide is 8754 dis h-1. The half life of Sr9038 is 27.7 years. Estimate the number of Sr9038 atoms in the sample at this time.
Note that there are 365.25 days in a year and 24 hours in a day.
Multiplying the number of days and years gives us 8765.81 hours per year.
Q19.3
What is the half-life of an isotope that is 75% decayed after 16 days?
If 75% has been used, 25% remains. That is to say, only 1/4 of it remains.
Therefore, it has gone through two half-lives.
\(\mathrm{\frac{1}{4}}\) = \(\mathrm{\frac {1}{2]}^n}\)
Hence,
n=2.
Q21.3.2
How does a star produce such enormous amounts of heat and light? How are elements heavier than Ni formed?
Stars produce enormous amounts of heat and light through nuclear fusion reactions. Small nuclei are "jammed together to form larger nuclei". That is to say, enough protons can collide into each other with enough speed that they stick together to form a helium nucleus and thus they generate a lot of heat and light. The binding energy holding the nuclei together is less in the heavier nuclides than in the original nuclides. Elements heavier than Ni are formed in super novae explosions.
Corrected my Max Win