Extra Credit 27
- Page ID
- 83536
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q19.9B
Calculate \(\mathrm{E_{cell}}\) using the Nernst equation for the following cells.
- \(\mathrm{Zn(s) | Zn^{2+}(aq)(0.1\,M) || Sn^{2+}(aq)(.8\,M) | Sn(s)}\)
- \(\mathrm{Cu(s) | Cu^{+}(aq)(0.4\,M) || F^{-}(aq)(O.9\,M) | F_2(g)(0.5\,atm) | Pt(s)}\)
S19.9B
Step 1: Make half reactions and determine the oxidation and reduction equation. (Make sure equation is balanced!)
Step 2: Find the E° for each half reactions using the standard reduction potential table.
Step 3: Add the E° of both half reactions together to find the E°cell.
Step 4: Determine the number of moles of electrons transferred (n).
Step 5: Calculate the reaction quotient (Q). \(Q= \frac{[Products]}{[Reactants]}\)
Step 6: Calculate the Ecell (Plug and chug). Ecell = E°cell - \(\frac{0.0592V}{n}\)logQ at 298K.
a) \(\mathrm{Zn(s) | Zn^{2+}(aq)(0.1\,M) || Sn^{2+}(aq)(.8\,M) | Sn(s)}\)
ox: Zn(s) → Zn2+(aq) + 2e- E° : -0.763
Red: Sn2+(aq) + 2e- → Sn(s) E° : -0.137
E°cell : -0.9
n=2
\(Q = \frac{[Zn^{2+}]}{[Sn^{2+}]} = \frac{0.1}{0.8} = 0.125\)
Ecell = E°cell - \(\frac{0.0592V}{n}\)logQ = -0.9 - \(\frac{0.0592V}{n}\)log(0.125)
Ecell = -0.8733V
b) \(\mathrm{Cu(s) | Cu^{+}(aq)(0.4\,M) || F^{-}(aq)(O.9\,M) | F_2(g)(0.5\,atm) | Pt(s)}\)
Cu(s) → Cu+(aq) + e- E° = -0.340
2F-(aq) → F2(g) + 2e- E° = -2.866
E°cell = 2.186
n=2
\(Q = \frac{[Cu^{2+}]}{[F^{-}]} = \frac{0.4}{0.9} = 0.4444\)
Ecell = E°cell - \(\frac{0.0592V}{n}\)logQ
= 2.186 - \(\frac{0.0592V}{n}\)log(0.4444)
Ecell = 2.196V
Q19.72A
Consider the two following reduction half reactions:
\[\mathrm{Sn^{4+} +2e^- \rightarrow Sn^{2+}}\]
\[\mathrm{Sn^{2+} +2e^- \rightarrow Sn(s)}\]
Calculate the \(\mathrm{E^\circ_{reduction}}\) for the reaction
\[\mathrm{Sn^{4+} +4e^- \rightarrow Sn (s)}\]
S19.72A
We will need to combine these equations, but we can’t simply add the \(\mathrm{E^\circ_{cell}}\) values together. We will need to convert and find the \(\mathrm{\Delta G}\) values for each equation. First, find the \(\mathrm{E^\circ_{cell}}\) of the two given half reactions: they are 0.154 V, and -0.137 V respectively from Table P2.
The \(\mathrm{\Delta G}\) of the first equation
\[ \mathrm{\Delta G^{\circ}} = \mathrm{n\, F \, E^{\circ}_{cell}}\]
Plugging in the values given will simplify to
\[\mathrm{ = -2 \, F \, (0.154) }\]
\[ \mathrm{\Delta G^{\circ}} = \mathrm{2 \, F \, (0.154)}\]
where \(\ce{F}\) is Faraday's constant.
2nd equation \(\mathrm{ = -2 ^* F ^* (-0.137) }\)
\(\mathrm{\Delta G}\) for the desired equation \(\mathrm{= (-0.308F) V + (0.274F)V}\)
\(\mathrm{\Delta G = (-0.034F)V}\)
Now to find the \(\mathrm{E^{\circ}_{cell} = \left (\dfrac{(-0.034F)V}{4 F} \right )\, ^* \,V = -0.0085\:V = E^{\circ}_{cell}}\)
For review on this topic, visit the page "Electrochemistry 5: Applications of the Nernst Equation".
Q21.5D
Draw Lewis structures for the following ligands:
- \(\ce{NH3}\)
- \(\ce{en}\)
- \(\ce{SCN-}\)
- \(\ce{NO2-}\)
S21.5D
Step 1: Find the total number of valence electrons.
Step 2: Elements up to period 4 generally follow the "Octet Rule," meaning they need 8 electrons to fill their outer shell.
Step 3: Determine the central atom. It is usually the atom with the highest valence or the least electronegative.
Step 4: Place electrons on the outside atoms first, the remaining electrons go on the central atom (creating lone pairs and/or double bonds, triple bonds, etc).
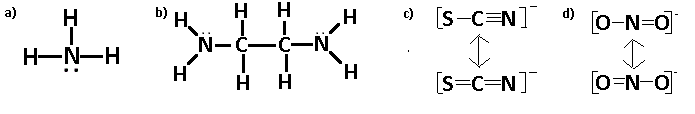
To review Lewis structures visit "Lewis Structures"
Q24.13D
Use the date table below to determine the rate law of the reacton: \(\ce{A + B \rightarrow 2D}\)
| Experiment | Initial \(\ce{[A]}\),M | Initial \(\ce{[B]}\),M | Initial Rate of Reaction, Ms-1 |
| 1 | 1.5 | 1.5 | 4.3 × 10-3 |
| 2 | 1.5 | 3.0 | 8.6 × 10-3 |
| 3 | 3.0 | 3.0 | 1.12 × 10-2 |
S24.13D
For more help see: The Rate of a Chemical Reaction
From looking at the table, \(\ce{[A]}\) is first order and \(\ce{[B]}\) is first order
overall rxn is 2nd order
\(\mathrm{R= k[A][B]}\)
\(\mathrm{4.3E\textrm{-3}\,Ms^{-1}= k(1.5\,M)(1.5\,M)}\)
\(\mathrm{k=1.2E\textrm{-3}\,(M^{-1})(s^{-1})}\)
Q24.61A
Please answer the following about the catalytic activity of both platinum metal and enzymes:
- Where are there active sites?
- Are they heterogeneous or homogeneous?
- Are they specific or nonspecific?
S24.61A
- The active site of platinum and of enzymes are at a metal center.
- Enzymes are usually homogeneous, meaning they are soluble in the reactant; platinum, however, is heterogeneous, meaning it cannot be dissolved in the reactant.
- Enzymes are extremely specific to their substrates, while platinum is usually more nonspecific.
Q25.29C
A science fiction writer is writing a short story about a team of scientists landing on Jupiter's moon for the first time. In their exploration, they find a large obelisk with some kind of electromagnetic field powered by an ancient fission reactor. Since the scientists' space suits already protect against the harmful radiation of interplanetary space, they decide to collect a sample and use it to find an approximate absolute age for the ship. The scientists determine that the reactor was able to run efficiently using proactinium-231 as fuel (remember that this is, indeed, fiction). If the scientists report to mission control that the reactor has been running for 30,000 years, what ratio of proactinium-231 to Uranium-235 and its derivatives was observed in the fictional field laboratory?
S25.29C
The half life of Proactinium-231 is 32,760 years. We use \(\mathrm{\lambda = \dfrac{0.693}{t_{1/2}}}\) to determine the rate constant of the reaction.
\[\mathrm{\lambda = \dfrac{0.693}{32,760\, y} = 2.11\times10^{-5}\, y^{-1}}\]
Now, using the equation: \(\mathrm{\ln\left(\dfrac{N_t}{N_0}\right) = - \lambda t}\), we can determine the ratio of moles of the protactinium-231 to Uranium-235 (and its derivatives)
\(\mathrm{\ln\left(\dfrac{N_t}{N_0}\right) = - \lambda t \Rightarrow \left(\dfrac{N_t}{N_0}\right) = e ^{- \lambda t} \Rightarrow \left(\dfrac{N_t}{N_0}\right) = e^{-(2.11\times10\textrm{E-005})(30,000)} = 0.53}\)
Knowing that the molar ratio of Proactinium-231 to Uranium-235 (and derivatives) is 0.53, we can determine the mass ratio of the sample collected fictional scientists.
Since there is a ratio of 0.53, that means there is 0.53 proactinium-231 moles per 1 mole of sample and conversely that there are 0.47 moles of Uranium-235 and its derivatives for every 1.00 mole of sample.
So, using some conversion factors for the molar mass of the species, we can determine that the ratio of Proactinium-231 to Uranium-235 was:
\(\mathrm{\left[\dfrac{\textrm{0.47 moles U-235}}{\textrm{0.53 moles Pa-231}}\right] \times \left[\dfrac{\textrm{235 g of U-235}}{\textrm{1 mole of U-235}}\right] \times \left[\dfrac{\textrm{1 mole of Pa-231}}{\textrm{231 g Pa-231}}\right] = \dfrac{\textrm{110.45 g U-235}}{\textrm{122.43 g Pa-231}} = \dfrac{\textrm{1 g U-235}}{\textrm{1.108 g Pa-231}}}\).
If the fictional Scientists reported that the artifact they found was 30,000 years old, they would have found 1 g of U-235 and its derivatives for every 1.108 g of Pa-231 fuel in the reactor.
Q21.1.6
List the three primary sources of naturally occurring radiation. Explain the factors that influence the dose that one receives throughout the year. Which is the largest contributor to overall exposure? Which is the most hazardous?
S21.1.6
Three primary naturally occurring radiations are radium, uranium and thorium, each all having long half lives. Inhalation of air, ingestion of food and water,terrestrial radation from the ground and cosmic radiation from space are all factors tat influence the does that a person receives throughout the year. Inhalation of the air is the largest contributor to exposure. Radiation can damage DNA or kill cells. When radiation is exposed to your body, it will collide with atoms and this will change and damage your DNA.
Q24.6.3
Will the value of Δo increase or decrease if I− ligands are replaced by NO2− ligands? Why?
S24.6.3
The value of Δo would increase because NO2− is a stronger ligand which means that it would have a larger split.
Review oxidation state for more help.

