Extra Credit 26
- Page ID
- 83535
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q19.9A
A voltaic cell with Ecell=1.500V has what [Ag+] in the cell?
Zn(s)|Zn2+(1.50M)||Ag+(?M)|Ag(s)
S19.9A
Solve for Ecell
Zn(s)→ Zn2+ + 2e- (oxidation) - anode E∘ = -0.763V
[Ag+ + e-→ Ag(s)] x 2 (reduction) - cathode E∘ = 0.800V
Zn(s)+2Ag+(aq)→Zn2+(aq)+2Ag(s)
E∘ cell = cathode - anode
E∘ cell= 0.800V - (-0.763V ) = 1.563V
Use the Nernst Equation
E=E∘cell−(0.05922/n) logQ
1.5V=1.563V−0.02961 log ([1.50]/[Ag+]2)
2.128 = log ([1.50]/[Ag+]2) --> 10(2.128) = 10(log ([1.50]/[Ag+]))
134.17 = 1.5/[Ag+]2
[Ag+] = 0.1054
Q19.65C
Out of the reactions given below, which of the following can occur spontaneously only through electrolysis. For those requiring electrolysis, what is the minimum voltage required?
- I2(s)+Ni(s)→2I−+Ni2+
- Br2(aq)+2Fe2+→2Br−+2Fe3+
- Ni2++2Cl−(aq)→Ni(s)+Cl2(g)
- Cr2+(aq)+Fe3+(aq)→Cr3+(aq)+Fe2+(aq)
S19.65C
1. I2(s)+Ni(s)→2I−+Ni2+
I2(s)+ 2e−→2I− (reduction) (cathode) --> 0.535V
Ni(s)→Ni2+ + 2e− (oxidation) (anode) --> -0.257V
E∘ cell = cathode - anode = 0.535 - (-0.257) = 0.792V
E∘ cell is positive, so not spontaneous through electrolysis. The minimum voltage is 0.80V.
2. Br2(aq)+2Fe2+→2Br−+2Fe3+
Br2(aq) + 2e−→2Br− (reduction) (cathode) --> 1.065V
2Fe2+→ 6e−+ 2Fe3+ (oxidation) (anode) -->0.745V
E∘ cell = cathode - anode = 1.065V - (0.745) = 0.32V
E∘ cell is positive, so not spontaneous through electrolysis. The minimum voltage is 0.33
3. Ni2++2Cl−(aq)→Ni(s)+Cl2(g)
Ni2++2e−→Ni(s) (reduction) (cathode) --> -0.257V
2Cl−(aq)→ 2e−+Cl2(g) (oxidation) (anode) --> 1.358V
E∘ cell = cathode - anode = -0.257V -(1.358) = -1.615V
E∘ cell is negative, so it is spontaneous through electrolysis.
4. Cr2+(aq)+Fe3+(aq)→Cr3+(aq)+Fe2+(aq)
Fe3+(aq) + e−→Fe2+(aq) (reduction) (cathode) --> 0.771V
Cr2+(aq)→Cr3+(aq) + 3e− (oxidation) (anode) --> 0.424V
E∘ cell = cathode - anode = 0.771 -(0.424) = 1.195V
E∘ cell is positive, so not spontaneous through electrolysis. The minimum voltage is 1.20V
Q21.5C
Draw Lewis structures for the following ligands: a) OH−OH−b) NO−2NO2− c) NH3NH3 d) SO−4SO4− e) enen (hint: C2H8N2C2H8N2)
S21.5C
a) 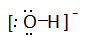
b) 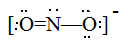
c) 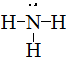
d) 
e) 
Q24.13C
The following rates of reactions were obtained in three experiments with the reaction 2NO(g)+Cl2(g)→2NOCl(g)2NO(g)+Cl2(g)→2NOCl(g)
|
Expt |
Initial [NO], M |
Initial [Cl2], M |
Initial rate of reaction, Ms-1 |
|---|---|---|---|
|
1 |
0.145 |
0.405 |
1.24 X 10-5 |
|
2 |
0.145 |
0.81 |
2.48 X 10-5 |
|
3 |
0.29 |
0.405 |
4.96 X 10-5 |
What is the rate law for this reaction?
S24.13C
[NO] 1/3 --> (0.145/0.29)x = (1.24 X 10-5/4.96 X 10-5) [Cl2] 1/2 --> (0.405/0.81)y = (1.24 X 10-5/2.48 X 10-5)
0.5x = 0.2419 0.5y = 0.5
x = 2 y = 1
r = k [NO]2[Cl2]
1.24 X 10-5 = k (0.1452)(0.405)
k = 1.46 X 10-3 M-2s-1
r = 1.46 X 10-3 M-2s-1 [NO]2[Cl2]
Q24.60B
The following substrates concentration [S] versus time date were obtained during an enzyme-catalyzed reaction: t = 0 min; [S] = 1.00M; 30 min, 0.90M; 90 min, 0.70M; 120 min, 0.50M; 180 min, 0.20M. What order is this reaction with respect to S in the concentration?
S24.60B
| time (min) | molarity (M) |
| 0 | 1 |
| 30 | 0.9 |
| 90 | 0.7 |
| 120 | 0.5 |
| 180 | 0.2 |
exponential decay, so it is first order
Q25.29B
Calculate the binding energy per nucleon, in megaelectronvolts, of the nuclide  if its measured mass is 28.1900 u.
if its measured mass is 28.1900 u.
Mass of proton=1.0073u
Mass of neutron=1.0087u
S25.29B
 → 14 protons + 16 neutrons
→ 14 protons + 16 neutrons
28.1900u → (14 x 1.0073u) + (16 x 1.0087u)
Δm = products - reactants = 30.2414 - 28.1900 = 2.0514u
1 u = 931.5MeV
2.0514u x (931.5MeV/u) = 1910.8791MeV/nuclei x (1 nuclei/30 nucleons) = 63.70MeV/nucleon
Q21.1.5
Gamma rays are a very high-energy radiation, yet α particles inflict more damage on biological tissue. Why?
S21.1.5
While gamma rays are high energy radiation and can pass through many materials, alpha particles are the most dangerous inside the body. Once inside the body, internal tissues absorb all of the particles' energy.
Q24.6.2
In CFT, what causes degenerate sets of d orbitals to split into different energy levels? What is this splitting called? On what does the magnitude of the splitting depend?
S24.6.2
The overlap of the orbitals and ligand fields disrupt the electrons, which causes the splitting. This splitting is t2g and eg .

