Extra Credit 46
- Page ID
- 82908
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.6.5
Why would a sacrificial anode made of lithium metal be a bad choice despite its E∘Li+/Li=−3.04VELi+/Li∘=−3.04V, which appears to be able to protect all the other metals listed in the standard reduction potential table?
S17.6.5
Although, the standard reduction potential of Lithium would indicate it has a high affinity towards being oxidized, the properties of this group 1 metal makes it a poor sacrificial anode. Although not the most reactive group 1 metal, Lithium is still a highly reactive element that has a tendency to catch on fire when in contact with water. Lithium is highly reactive due to it having only one valence electron. By only having one valence electron, said electron can be easily removed. And because elements tend to want to have completely filled shells rather than partially filled shells, Lithium reacts with other elements to remove the unwanted electron. This reactivity causes Lithium to be a bad choice as a sacrificial anode. Being reactive with almost all other elements causes Lithium to degrade rather quickly and would need to be replaced frequently. Also, Lithium poses a great safety hazard, due to it igniting spontaneously when in contact with any source of water. Below is a short video on just how reactive Lithium is to water!
Q12.3.9
What is the instantaneous rate of production of N atoms Q12.3.8 in a sample with a carbon-14 content of 1.5 × 10−9 M?
Q12.3.8 for reference
The rate constant for the radioactive decay of 14C is 1.21 × 10−4 year−1. The products of the decay are nitrogen atoms and electrons (beta particles):
\[ {^{6}_{14}}C \rightarrow {^{6}_{14}}N + e^- \]
\[ rate = k[{^{6}_{14}}C] \]
What is the instantaneous rate of production of N atoms in a sample with a carbon-14 content of 6.5 × 10−9 M?
S12.3.9
From this question, we are asked to determine the production rate of a decaying Carbon-14 sample of 1.5 x 10-9 Mol/L. Recall from section 14.3 that the reaction rate of a general equation can be determined from its reactant's concentrations, k constant, and reaction orders. Fortunately, we are given all of the essential information needed to solve for the reaction rate!
What is given:
- Rate Constant = 1.21 × 10−4 year−1
- The Rate Formula = ( rate = k[C-14] )
- Concentration = 1.5 × 10−9 M
To solve for the reaction rate we take the give Rate Formula and plug in the information given into said equation!
\[ rate = k[{^{6}_{14}}C] \]
\[ k = ({1.21} \times 10^{-4}\;year^{-1}) \]
\[ C = [1.5 \times 10^{-9} M] \]
\[ = ({1.21} \times 10^{-4}\;year^{-1})[1.5 \times 10^{-9} M] \]
\[ = ({1.82} \times 10^{-13}) \frac{M}{year}\]
Q12.6.1
Why are elementary reactions involving three or more reactants very uncommon?
S12.6.1
Reactions involving three reactants are rare due to the conditions needed to proceed with said reaction being difficult to meet. Recall from the Collision Theory that in order for a chemical reaction to occur reacting particles must collide with sufficient energy and with the proper orientation. Having three particles simultaneously collide with the correct orientation and sufficient energy is a very uncommon occurrence, thus, termolecular reactions are rare. An analogy of this idea is picking a number from 1 to 10. If you are able to select the correct number the "reaction" will proceed. Let's start off with only having to choose one correct number, with the correct choice being number 7:
(1, 2, 3, 4 ,5 ,6 ,7 ,8 ,9 ,10)
The odds of you picking the correct number is 1/10.
Now lets try to pick 3 correct numbers, with each number selection corresponding to different number sets. (The correct choices being 3,16, and 21)
(1, 2, 3, 4 ,5 ,6 ,7 ,8 ,9 ,10)
(11, 12, 13, 14 ,15 ,16 ,17 ,18 ,19 ,20)
(21, 22, 23, 24 ,25 ,26 ,27 ,28 ,29 ,30)
The odds of you picking the correct number is 1/1000, 100 times lower than only having to choose one correct number. This example mirrors the probability of three reactant particles colliding with one another, comparative to a reaction containing one reactant. The chances of getting the correct orientation and energy (mirrored by correct number) for the three reactants to collide is highly unlikely.
Q21.4.13
Define the term half-life and illustrate it with an example.
S21.4.13
Half-life refers to the amount of time needed for some initial reactant concentration amount to decrease by half. An example of this is a reaction involving the reactant IO3 - that starts with an initial concentration of 0.010 M.
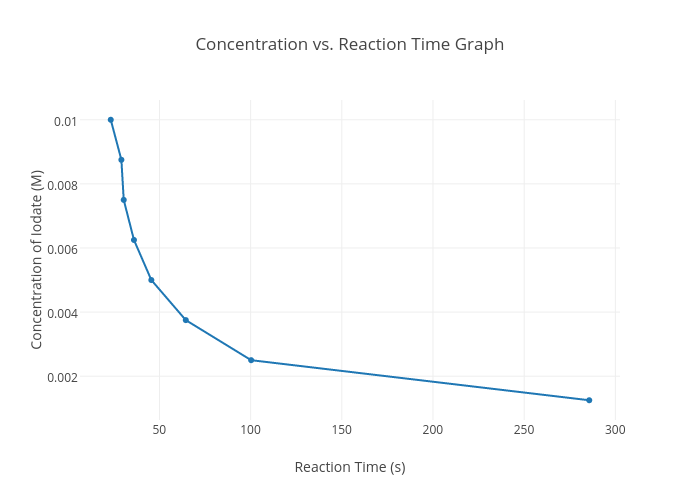
From the graph above, we are given the relationship between [IO3-] and time. To determine the half-life for this reaction we must first determine the time in which our initial concentration is halved. Referring back to the graph the IO3- reaches a concentration of 0.0050 M (half the initial concentration) around 50 seconds. Thus is can be concluded that the half-life of the reaction takes roughly about 50 seconds!
Q20.3.1
Is 2NaOH(aq)+H2SO4(aq)→Na2SO4(aq)+2H2O(l)2NaOH(aq)+H2SO4(aq)→Na2SO4(aq)+2H2O(l) an oxidation–reduction reaction? Why or why not?
S20.3.1
To determine if the given reaction is an oxidation-reduction reaction, recall that an oxidation-reduction (redox) reaction is a chemical reaction that involves the transfer of electrons between two species and the reduction (decrease in oxidation state) or oxidation (increase in oxidation state) of a(n) atoms/molecules/ion. The first step in determining if this is indeed a redox reaction is to find the oxidation number of each atom! If you have forgotten the rules to assigning oxidation numbers to click here!
Let's start off with the molecule on the reactant side!
\[ \text{2NaOH}(\mathit{aq}) + \text{H}_2\text{SO}_4(\mathit{aq}) \]
NaOH consist of three smaller subunits:
\[ \text{Na, O, and H} \]
Because Na belongs to the group 1 elements it is given an oxidation state of +1
\[ \text{Na}^+ \]
Hydrogen is given a +1 oxidation state as well
\[ \text{H}^+ \]
Since the original molecule is a neutral species, the positive charge from Na+ and H+ must be canceled. Thus the oxidation state of O must be -2
\[ \text{O}^{-2} \]
H2SO4 consist of three smaller subunits as well:
\[ \text{2H, S}\;\text{and}\;\text{O}_4 \]
Since there is Hydrogen in the compound it is given an oxidation state of +1
\[ \text{2H}^+ \]
Being that Oxygen is within the compound it is given an oxidation state of -2
\[ \text{O}^{-2} \]
In order for the compound to remain neutral the positive charge from H and negative charge from O must be canceled out by S. Since the 2 Hydrogens provide a +2 and the 4 Oxygens provide a -8, S must take on an oxidation state of +6.
\[ \text{S}^{+6} \]
Next lets examine the products!
\[ \text{Na}_2\text{SO}_4(\mathit{aq}) + \text{2H}_2\text{O}(\mathit{l}) \]
Breaking down Na2SO4 give us three small subunits:
\[ \text{2Na, S,} \;\text{and}\;\text{O}_4 \]
Because Na belongs to the group 1 elements it is given an oxidation state of +1
\[ \text{Na}^+ \]
And since there is an Oxygen in the compound, it is given a charge of -2
\[ \text{O}^{-2} \]
In order to make the compound neutral, the +2 charge contributed by the two Na atoms and -8 charge by the Oxygen atoms must be canceled by S giving it an oxidation state of +6
\[ \text{S}^{+6} \]
Doing the same thing to H2O we have:
\[ \text{2H and O} \]
Since there is a hydrogen in the compound, H is given the oxidation state of +1
\[ \text{H}^+ \]
And in order to ensure the compound is neutral, the +2 charge provided by the hydrogens needs to be canceled, thus Oxygen is given the oxidation state of -2
\[ \text{O}^{-2} \]
Since none of the molecules/atoms/ions lose or gain electrons, no reduction or oxidation has occurred. Thus the above reaction is not a redox reaction.
Q20.5.12
How many electrons are transferred during the reaction Pb(s) + Hg2Cl2(s) → PbCl2(aq) + 2Hg(l)? What is the standard cell potential? Is the oxidation of Pb by Hg2Cl2 spontaneous? Calculate ΔG° for this reaction.
S20.5.12
Since the question is asking for the transfer of electrons in a reaction, it is assumed the above reaction is a redox reaction. In order to determine the number of electrons transferred in the redox reaction, we must first determine the reduction and oxidation half-reactions.
Because Pb is changed from a neutral charge to an oxidation state of -2, the oxidation half-reaction is:
\[ \text{Pb}(\mathit{s}) \rightarrow \text{Pb}^{+2}(\mathit{aq}) + \text{2e}^-\]
And since the charge of Hg is changed from +1 to becoming netural, the reduction half-reaction is"
\[ \text{2Hg}^+(\mathit{aq}) + \text{2e}^- \rightarrow \text{2Hg}(\mathit{l}) \]
Seeing that the total number of electron is already balanced between the two half-reactions, the total number of electrons transferred is 2!
Now in order to solve the cell potential of the reaction let's pretend that this reaction is occurring in a Galvanic Cell. Recall that reduction occurs at the cathode and oxidation occurs at the anode. This allows us to infer that the reduction half-reaction found in above reacts at the cathode and the oxidation half-reaction occurs at the anode! Luckily, there is a formula that relates the standard reduction potential of the cathode and anode to give us the overall cell potential. The standard reduction potential for various species is found in the Activity Series.
The overall equation for determining the standard cell potential (E°cell) is:
\[\text{E}^°(\mathit{Cell}) = \text{E}^°(\mathit{Cathode})\;-\;\text{E}^°(\mathit{Anode}) \]
The standard reduction potential for the half-reactions determine beforehand are:
\[ \text{Cathode:}\;\text{2Hg}^+(\mathit{aq}) + \text{2e}^- \rightarrow \text{2Hg}(\mathit{l})\;\;\;\;\text{E}^°= 0.85 V \]
\[ \text{Anode:}\;\text{Pb}^{2+} + \text{2e}^{-} \rightarrow \text{Pb}(\mathit{s})\;\;\;\;\text{E}^°= -0.13 V \]
Plugging into the standard cell potential equation:
\[\text{E}^°(\mathit{Cell}) = (0.85V) - (-0.13 V) \]
\[ = (0.85V) + (0.13 V) \]
\[ = (0.85V) + (0.13 V) \]
\[ = 0.98V \]
To determine the spontaneous nature of the reaction above we can look to the cell potential calculated. A cell potential > 0 will details that the reaction is spontaneous, while a cell potential that is < 0 tells us the reaction is non-spontaneous.
Because the calculated value of the cell potential (from above) is positive, it can be said that the oxidation of Pb by Hg2Cl2 is spontaneous!
To ensure that the given reaction will be spontaneous let's determine ΔG. Recall that Cell Potential and Free Energy share a relationship together based on the amount of work (energy).
The equation relating ΔG to cell potential is:
\[ \Delta\text{G}^° = -\text{nFE}^°_{cell} \]
\[ \text{n = number of electrons} \]
\[ \text{F = Faraday's Constant = 96,486 (J)/(V)(mol e)} \]
The information to solve for ΔG has been determined from the before hand questions. Thus, the last steps into obtaining ΔG is to plug in the values into the equation!
\[ \Delta\text{G}^° = -\text{nFE}^°_{cell} \]
\[ = -(2)(96,486 J/V⋅mol)(0.98V) \]
\[ = -189113 J \]
Q24.6.8
For each complex, predict its structure, whether it is high spin or low spin, and the number of unpaired electrons present.
- [Cu(NH3)4]2+
- [Ni(CN)4]2−
S24.6.8
To be able to solve the above question, we must understand the Crystal Field Theory. In transition-metal complexes the ligands that are attached to the transition metal causes the breaking of the degeneracy in the d orbitals. This leads to the d-orbital energy-levels to split into an upper level (x2-y2,z2 orbitals) and lower level (xy,xz,yz), separated by Δo [This splinting is for octahedral complex only]. The method in which the levels split is determined primarily by the geometry of the complex and the strength of the ligands attached.
Looking at the complex given in the question, it can be seen that the possible geometry of the complexes can either be tetrahedral or square planar. This is for the number of ligands attached to the transition metal is four in both of the given complexes. The true geometry the complexes will take is the complex that would give the lowest net total energy. Figures for the square planar and tetrahedral d-orbital energy level diagrams can be found here!
Let's take a look at the complex [Cu(NH3)4]2+. In this complex, the central transition-metal has a d9 orbital. To figure out the structure it will take we must determine which geometry will result in the lowest net energy. And since this metal has 9 free electrons there is no high spin or low spin possible and will only have one unpaired electron, thus only two possibilities exist!
\[ \text{Energy}(\mathit{Tetrahedral}) = (-3/5\Delta o)(A) + (2/5\Delta o)(B) + (Z)P \]
\[ \text{A = The Number of Electrons in the x}^2-\text{y}^2,\text{z}^2\; \text{orbitals} \]
\[ \text{B = The Number of Electrons in the xy, xz, and yz orbitals} \]
\[ \text{Z = The Number of paired Electrons} \]
\[ \text{Energy}(\mathit{Tetrahedral}) = (-3/5\Delta o)(4) + (2/5\Delta o)(5) + (4)P \]
\[ = (-12/5\Delta o) + (2\Delta o) + (4P) \]
\[ = -0.4\Delta o + 4P \]
\[ \text{Energy}(\mathit{Square Planar}) = (-0.51\Delta o)(A) + (-0.43\Delta o)(B) + (0.23\Delta o)(C) + (1.23\Delta o)(D) + (Z)P \]
\[ \text{A = The Number of Electrons in the xz and yz orbitals} \]
\[ \text{B = The Number of Electrons in the}\; \text{z}^2\; \text{orbital} \]
\[ \text{C = The Number of Electrons in the xy orbital} \]
\[ \text{D = The Number of Electrons in the x}^2-\text{y}^2\; \text{orbital} \]
\[ \text{Z = The Number of paired Electrons} \]
\[ \text{Energy}(\mathit{Square Planar}) = (-0.51\Delta o)(4) + (-0.43\Delta o)(2) + (0.23\Delta o)(2) + (1.23\Delta o)(1) + (4)P \]
\[ = (-2.04\Delta o) + (-0.86\Delta o) + (0.46\Delta o) + (1.23\Delta o) + (4P) \]
\[ = -1.21\Delta o + 4P \]
\[ -1.21\Delta o + 4P < -0.4\Delta o + 4P \]
Because the square planar geometry has a lower total energy than the tetrahedral structure, the most likely structure [Cu(NH3)4]2+ will take is a square planar.
[Cu(NH3)4]2+ has a square planar geometry, neither a high spin or low spin state, and has 1 unpaired electron!
Using the same method above, the structure of [Ni(CN)4]2− can be determined. However because the metal ion is a d8 , high spin and low spin states are possible. To determine the correct geometry and spin state calculate the net splitting energy and the lowest value will detail the structure and spin state. You should have four total splitting energies by the end (one for each structure and spin state). After you have calculated all the splitting energies, the lowest one should determine that:
[Ni(CN)4]2− has a square planar geometry, a low spin state, and has no unpaired electrons!
Q14.7.12
A particular reaction has two accessible pathways (A and B), each of which favors conversion of X to a different product (Y and Z, respectively). Under uncatalyzed conditions pathway A is favored, but in the presence of a catalyst pathway B is favored. Pathway B is reversible, whereas pathway A is not. Which product is favored in the presence of a catalyst? without a catalyst? Draw a diagram illustrating what is occurring with and without the catalyst.
S14.7.12
The first step to solving this question is to determine what information is given to us. What we know is that there are two pathways for the conversion of X to a different product. Under uncatalyzed conditions, pathway A is favored and X with be converted to Y. In catalyzed conditions pathway B is favored, X will be converted into Z, and the reaction becomes reversible. Let right down the equations that describe the given information!
\[ \text{Pathway A:} \;X \rightarrow Y \]
\[ \text{Pathway B:} \; X \leftrightarrow Z \]
From here a relationship between the two pathways can be created
\[ \text{Pathway A and B:} \; Z \leftrightarrow X \rightarrow Y \]
By looking at the relationship between the two pathways it can be seen that the product Y will be favored in all conditions. If the reaction proceeds under uncatalyzed conditions X will be only converted to Y. If the reaction takes places in catalyzed conditions Z and X will both be produced. However, the X is also converted to Y at the same time and eventually because of the reversibility of Z to X, all X will eventually be turned into Y.
In both catalyzed and uncatalyzed conditions, the product that will be favored is Y (Pathway A's product)

