3.11: Synthesis of Polysubstituted Benzenes
- Page ID
- 500379
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- Design a multistep synthesis which may involve reactions in the alkyl side chain of an alkylbenzene and the electrophilic substitution reactions discussed in this chapter. You should pay particular attention to
- carrying out the reactions in the correct order.
- using the most appropriate reagents and conditions.
- the limitations of certain types of reactions.
- Analyze a proposed multistep synthesis involving aromatic substitution to determine its feasibility, point out any errors in the proposal and identify possible problem areas.
As discussed in the Introduction to Organic Synthesis, one of the surest ways to learn organic chemistry is to work synthesis problems. The ability to plan a successful multistep synthesis of a complex molecule requires a working knowledge of the uses and limitations of a great many organic reactions. Not only must you know which reactions to use, you must also know when to use them because the order in which reactions are carried out is often critical to the success of the overall scheme.
The ability to plan a sequence of reactions in the right order is particularly important in the synthesis of substituted aromatic rings, where the introduction of a new substituent is strongly affected by the directing effects of other substituents. Planning syntheses of substituted aromatic compounds is therefore a good way to gain confidence in using the many reactions discussed in the past few chapters.
It’s usually best to work a synthesis problem backward, or retrosynthetically. Look at the target molecule and ask yourself, “What is an immediate precursor of this compound?” Choose a likely answer and continue working backward, one step at a time, until you arrive at a simple starting material. Let’s try some examples.
From benzene make m-bromoaniline
In this reaction three reactions are required.
- A nitration
- A conversion from the nitro group to an amine
- A bromination
Because the end product is meta a meta directing group must be utilized. Of the nitro, bromine, and amine group, only the nitro group is meta direction. This means that the first step need to be the nitration and not the bromination. Also, the conversion of the nitro group to an amine must occur last because the amine group is ortho/para direction.
From benzene make p-nitropropylbenzene :
In this reaction three reactions are required.
- A Friedel Crafts acylation
- A conversion from the acyl group to an alkane
- A nitration
Because the propyl group has more than two carbons, it must be added in two steps. A Friedel Crafts acylation followed by a Clemmensen Reduction. Remember that Friedel Crafts reactions are hindered if the benzene ring is strongly deactivated. This means that the acyl group must go on first. Because the end product is para a para directing group must be utilized. Of the nitro, acyl, and alkane group, only the alkane group is meta direction. This means that the acyl group must be converted to an alkane prior to the nitration step.
How would you synthesize 4-bromo-2-nitrotoluene from benzene?
Strategy
Draw the target molecule, identify the substituents, and recall how each group can be introduced separately. Then plan retrosynthetically.

The three substituents on the ring are a bromine, a methyl group, and a nitro group. A bromine can be introduced by bromination with Br2/FeBr3, a methyl group can be introduced by Friedel–Crafts alkylation with CH3Cl/AlCl3, and a nitro group can be introduced by nitration with HNO3/H2SO4.
Solution
Ask yourself, “What is an immediate precursor of the target?” The final step will involve introduction of one of three groups—bromine, methyl, or nitro—so we have to consider three possibilities. Of the three, the bromination of o-nitrotoluene could be used because the activating methyl group would dominate the deactivating nitro group and direct bromination to the correct position. Unfortunately, a mixture of product isomers would be formed. A Friedel–Crafts reaction can’t be used as the final step because this reaction doesn’t work on a nitro-substituted (strongly deactivated) benzene. The best precursor of the desired product is probably p-bromotoluene, which can be nitrated ortho to the activating methyl group to give a single product.

Next ask, “What is an immediate precursor of p-bromotoluene?” Perhaps toluene is an immediate precursor because the methyl group would direct bromination to the ortho and para positions. Alternatively, bromobenzene might be an immediate precursor because we could carry out a Friedel–Crafts methylation and obtain a mixture of ortho and para products. Both answers are satisfactory, although both would also lead unavoidably to a mixture of products that would have to be separated.
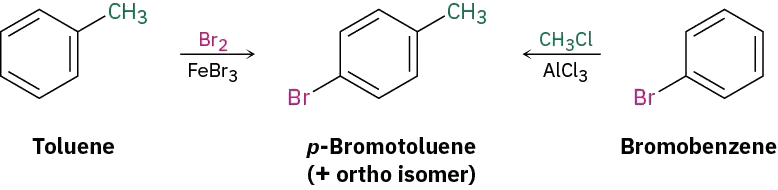
“What is an immediate precursor of toluene?” Benzene, which could be methylated in a Friedel–Crafts reaction. Alternatively, “What is an immediate precursor of bromobenzene?” Benzene, which could be brominated.
The retrosynthetic analysis has provided two valid routes from benzene to 4-bromo-2-nitrotoluene.

Synthesize 4-chloro-2-propylbenzenesulfonic acid from benzene.
Strategy
Draw the target molecule, identify its substituents, and recall how each of the three can be introduced. Then plan retrosynthetically.

The three substituents on the ring are a chlorine, a propyl group, and a sulfonic acid group. A chlorine can be introduced by chlorination with Cl2/FeCl3, a propyl group can be introduced by Friedel–Crafts acylation with CH3CH2COCl/AlCl3 followed by reduction with H2/Pd, and a sulfonic acid group can be introduced by sulfonation with SO3/H2SO4.
Solution
“What is an immediate precursor of the target?” The final step will involve introduction of one of three groups—chlorine, propyl, or sulfonic acid—so we have to consider three possibilities. Of the three, the chlorination of o-propylbenzenesulfonic acid can’t be used because the reaction would occur at the wrong position. Similarly, a Friedel–Crafts reaction can’t be used as the final step because this reaction doesn’t work on sulfonic-acid-substituted (strongly deactivated) benzenes. Thus, the immediate precursor of the desired product is probably m-chloropropylbenzene, which can be sulfonated to give a mixture of product isomers that must then be separated.

“What is an immediate precursor of m-chloropropylbenzene?” Because the two substituents have a meta relationship, the first substituent placed on the ring must be a meta director so that the second substitution will take place at the proper position. Furthermore, because primary alkyl groups such as propyl can’t be introduced directly by Friedel–Crafts alkylation, the precursor of m-chloropropylbenzene is probably m-chloropropiophenone, which could be catalytically reduced.

“What is an immediate precursor of m-chloropropiophenone?” Propiophenone, which could be chlorinated in the meta position.

“What is an immediate precursor of propiophenone?” Benzene, which could undergo Friedel–Crafts acylation with propanoyl chloride and AlCl3.

The final synthesis is a four-step route from benzene:

How would make the following compounds from benzene?
- m-bromonitrobenzene
- m-bromoethylbenzene
- 4-Chloro-1-nitro-2-propylbenzene
- 3-Bromo-2-methylbenzenesulfonic acid
- Answer
-
Only one possible synthesis is shown for each compound. There are multiple possibilities.
C) 1. CH3CH2COCl, AlCl3; 2. Cl2, FeCl3; 3. H2/Pd; 4. HNO3, H2SO4
D) 1. CH3Cl, AlCl3; 2. Br2, FeBr3; 3. SO3, H2SO4
In planning a synthesis, it’s as important to know what not to do as to know what to do. As written, the following reaction schemes have flaws in them. What is wrong with each?
a.
b. 
c. 
- Answer
-
- The bromine should be in the meta position. Right now it is in the ortho position, from perhaps having the ethyl group present first and then the having it substituted there. BUT the ethyl group is last to form, and the aldehyde and nitro groups would both encourage a meta substitution.
- Friedel–Crafts acylation does not occur on a deactivated ring.
- Rearrangement occurs during Friedel–Crafts alkylation with primary halides; chlorination occurs ortho to the alkyl group.


