1.1: Introduction to Chemistry
- Page ID
- 360558
- Distinguish beween chemistry and physics;
- Suggest ways in which the fields of engineering, economics, and geology relate to Chemistry;
- Define the following terms, and classify them as primarily microscopic or macroscopic concepts: element, atom, compound, molecule, formula, structure.
Chemistry is a very universal and dynamically-changing subject to be confined to a fixed definition; it might be better to think of chemistry more as a point of view that places its major focus on the structure and properties of substances— particular kinds of matter— and especially on the changes they undergo.
The real importance of Chemistry is that it serves as the interface to practically all of the other sciences, as well as to many other areas of human endeavor. For this reason, Chemistry is often said (at least by chemists!) to be the "central science". Chemistry can be "central" in a much more personal way: with a solid background in Chemistry, you will find it far easier to migrate into other fields as your interests develop.
Research or teaching not for you? Chemistry is so deeply ingrained into so many areas of business, government, and environmental management that some background in the subject can be useful (and able to give you a career edge as a team member having special skills) in fields as varied as product development, marketing, management, computer science, technical writing, and even law.
So just what is chemistry?
Do you remember the story about the group of blind men who encountered an elephant? Each one moved his hands over a different part of the elephant's body— the trunk, an ear, or a leg— and came up with an entirely different description of the beast. Chemistry can similarly be approached in different ways, each yielding a different, valid, and yet hopelessly incomplete view of the subject. Thus we can view chemistry from multiple standpoints ranging from the theoretical to the eminently practical:
| Mainly theoretical | Mainly practical |
|---|---|
| Why do particular combinations of atoms hold together, but not others? | What are the properties of a certain compound? |
| How can I predict the shape of a molecule? | How can I prepare a certain compound? |
| Why are some reactions slow, while others occur rapidly? | Does a certain reaction proceed to completion? |
| Is a certain reaction possible? | How can I determine the composition of an unknown substance? |
Chemistry is the study of substances; their properties, structure, and the changes they undergo.
Micro-macro: the forest or the trees
Chemistry, like all the natural sciences, begins with the direct observation of nature — in this case, of matter. But when we look at matter in bulk, we see only the "forest", not the "trees" — the atoms and molecules of which matter is composed of — whose properties ultimately determine the nature and behavior of the matter we are looking at. This dichotomy between what we can and cannot directly see constitutes two contrasting views which run through all of chemistry, which we call macroscopic and microscopic.
In the context of Chemistry, "microscopic" implies the atomic or subatomic levels which cannot be seen directly (even with a microscope!) whereas "macroscopic" implies things that we can know by direct observations of physical properties such as mass, volume, etc. The following table provides a conceptual overview of Chemical science according to the macroscopic/microscopic dichotomy we have discussed above. It is of course only one of the many ways of looking at the subject, but you may find it helpful in organizing the many facts and ideas that you will encounter in your study of Chemistry. We will organize the discussion in this lesson along similar lines.
|
realm |
macroscopic view |
microscopic view |
|---|---|---|
| composition | formulas, mixtures | structures of solids, molecules, and atoms |
| properties | intensive properties of bulk matter | particle sizes, masses and interactions |
| change (energetics) | energetics and equilibrium | statistics of energy distribution |
| change (dynamics) | kinetics (rates of reactions) | mechanisms |
Chemical composition
Mixture or "pure substance" ?
In science it is necessary to know what we are talking about, so before we can begin to consider matter from a chemical point of view, we need to know its composition; whether it is a single substance, or a mixture? (We will get into the details of the definitions later, but for the moment you probably have a fair understanding of the distinction; think of a sample of salt (sodium chloride) as opposed to a solution of salt in water— a mixture of salt and water.)
Elements and compounds
It has been known for at least a thousand years that some substances can be broken down by heating or chemical treatment into "simpler" ones, but there is always a limit; we eventually get substances known as elements that cannot be reduced to any simpler forms by ordinary chemical or physical means. What is our criterion for "simpler"? The most observable (and therefore macroscopic) property is the weight.
The idea of a minimal unit of chemical identity that we call an element developed from experimental observations of the relative weights of substances involved in chemical reactions. For example, the compound mercuric oxide can be broken down by heating into two other substances:
\[\ce{2 HgO \rightarrow 2 Hg + O_2}\]
... but the two products, metallic mercury and dioxygen, cannot be decomposed into simpler substances, so they must be elements.
Elements and atoms
The definition of an element given above is an operational one; a certain result (or in this case, a non-result!) of a procedure that might lead to the decomposition of a substance into lighter units will tentatively place that substance into one of the categories: element or compound. Because this operation is carried out on bulk matter, the concept of the element is also a macroscopic one. The atom, by contrast, is a microscopic concept which in modern chemistry relates the unique character of every chemical element to an actual physical particle.
The idea of the atom as the smallest particle of matter had its origins in Greek philosophy around 400 BCE but was controversial from the start (both Plato and Aristotle maintained that matter was infinitely divisible.) It was not until 1803 that John Dalton proposed a rational atomic theory to explain the facts of chemical combination as they were then known, thus being the first to employ macroscopic evidence to illuminate the microscopic world. It wasn't until the 1900s that the atomic theory became universally accepted. In the 1920's it became possible to measure the sizes and masses of atoms, and in the 1970's techniques were developed that produced images of individual atoms.

Formula and structure
The formula of a substance expresses the relative number of atoms of each element it contains. Because the formula can be determined by experiments on bulk matter, it is a macroscopic concept even though it is expressed in terms of atoms.
What the ordinary chemical formula does not tell us is the order in which the component atoms are connected, whether they are grouped into discrete units (molecules) or are two- or three dimensional extended structures, as is the case with solids such as ordinary salt. The microscopic aspect of composition is the structure, which gives detailed relative locations (in two or three dimensional space) of each atom within the minimum collection needed to define the structure of the substance.
|
|
Macroscopic |
Microscopic |
|---|---|---|
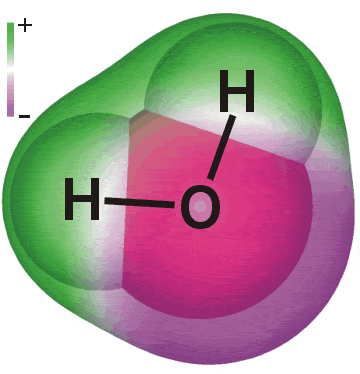
| Substances are defined at the macroscopic level by their formulas or compositions, and at the microscopic level by their structures. | The elements hydrogen and oxygen combine to form a compound whose composition is expressed by the formula H2O. | The molecule of water has the structure shown here.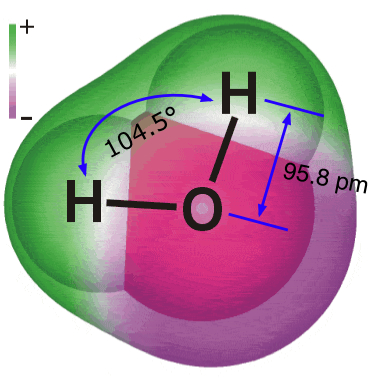 |
| Chemical substances that cannot be broken down into simpler ones are known as elements. The actual physical particles of which elements are composed are atoms or molecules. |
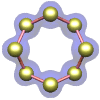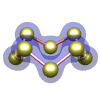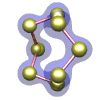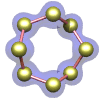
Sulfur – the element in its orthorhombic crystalline form.
|
molecule is an octagonal ring of sulfur atoms. The crystal shown at the left is composed of an ordered array of these molecules.
(No, they don't actually move around like this, although they are in a constant state of vibrational motion.)
|
Compounds and molecules
As we indicated above, a compound is a substance containing more than one element. Since the concept of an element is macroscopic and the distinction between elements and compounds was recognized long before the existence of physical atoms was accepted, the concept of a compound must also be a macroscopic one that makes no assumptions about the nature of the ultimate. Thus when carbon burns in the presence of oxygen, the product carbon dioxide can be shown by (macroscopic) weight measurements to contain both of the original elements:
\[\ce{C + O2 -> CO2}\]
10.0 g + 26.7 g = 36.7 g
One of the important characteristics of a compound is that the proportions by weight of each element in a given compound are constant. For example, no matter what weight of carbon dioxide we have, the percentage of carbon it contains is (10.0 / 36.7) = 0.27, or 27%.
Molecules
A molecule is an assembly of atoms having a fixed composition, structure, and distinctive, measurable properties.
"Molecule" refers to a kind of particle, and is therefore a microscopic concept. Even at the end of the 19th century, when compounds and their formulas had long been in use, some prominent chemists doubted that molecules (or atoms) were any more than a convenient model.
Molecules suddenly became real in 1905, when Albert Einstein showed that Brownian motion, the irregular microscopic movements of tiny pollen grains floating in water, could be directly attributed to collisions with molecule-sized particles.
Finally, we get to see one!
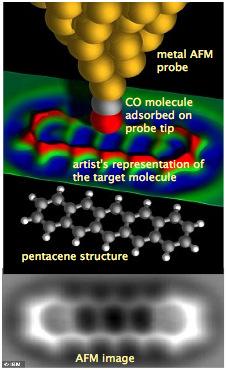
In 2009, IBM scientists in Switzerland succeeded in imaging a real molecule, using a technique known as atomic force microscopy in which an atoms thin metallic probe is drawn ever-so-slightly above the surface of an immobilized pentacene molecule cooled to nearly absolute zero. In order to improve the image quality, a molecule of carbon monoxide was placed on the end of the probe.
The image produced by the AFM probe is shown at the very bottom. What is actually being imaged is the surface of the electron clouds of the molecule, which consists of six hexagonal rings of carbon atoms with hydrogen on its periphery. The tiny bumps that correspond to these hydrogen atoms attest to the remarkable resolution of this experiment.
The atomic composition of a molecule is given by its formula. Thus the formulas CO, CH4, and O2 represent the molecules carbon monoxide, methane, and dioxygen. However, the fact that we can write a formula for a compound does not imply the existence of molecules having that composition. Gases and most liquids consist of molecules, but many solids exist as extended lattices of atoms or ions (electrically charged atoms or molecules.) For example, there is no such thing as a "molecule" of ordinary salt, NaCl (see below.)
Confused about the distinction between molecules and compounds?
Maybe the following will help:
 |
  |
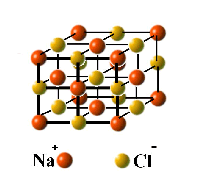 |
| A molecule but not a compound - Ozone, O3, is not a compound because it contains only a single element. | This well-known molecule is a compound because it contains more than one element. | Ordinary solid salt is a compound but not a molecule. It is built from interpenetrating lattices of sodium and chloride ions that extend indefinitely. |
Structure and properties
Composition and structure lie at the core of Chemistry, but they encompass only a very small part of it. It is largely the properties of chemical substances that interest us; it is through these that we experience and find uses for substances, and much of chemistry as a science is devoted to understanding the relation between structure and properties. For some purposes it is convenient to distinguish between chemical properties and physical properties, but as with most human-constructed dichotomies, the distinction becomes more fuzzy as one looks more closely.
Chemical change
Chemical change is defined macroscopically as a process in which new substances are formed. On a microscopic basis it can be thought of as a re-arrangement of atoms. A given chemical change is commonly referred to as a chemical reaction and is described by a chemical equation that has the form
reactants → products
In elementary courses it is customary to distinguish between "chemical" and "physical" change, the latter usually relating to changes in physical state such as melting and vaporization. As with most human-created dichotomies, this begins to break down when examined closely. This is largely because of some ambiguity in what we regard as a distinct "substance".
Elemental chlorine exists as the diatomic molecule \(\ce{Cl2}\) in the gas, liquid, and solid states; the major difference between them lies in the degree of organization. In the gas the molecules move about randomly, whereas in the solid they are constrained to locations in a 3-dimensional lattice. In the liquid, this tight organization is relaxed, allowing the molecules to slip and slide around each other.
Since the basic molecular units remain the same in all three states, the processes of melting, freezing, condensation and vaporization are usually regarded as physical rather than chemical changes.
Solid salt consists of an indefinitely extended 3-dimensional array of Na+ and Cl– ions (electrically charged atoms.)
When heated above 801°C, the solid melts to form a liquid consisting of these same ions. This liquid boils at 1430° to form a vapor made up of discrete molecules having the formula \(\ce{Na2Cl2 (aq)}\).. Because the ions in the solid, the hydrated ions in the solution, and the molecule \(\ce{Na2Cl2}\) are really different chemical species, so the distinction between physical and chemical change becomes a bit fuzzy.
Currents of modern Chemistry
In the preceding section we looked at chemistry from a conceptual standpoint. If this can be considered a "macroscopic" view of chemistry, what is the "microscopic" view? It would likely be what chemists actually do. Because a thorough exploration of this would lead us into far more detail than we can accommodate here, we will mention only a few of the areas that have emerged as being especially important in modern chemistry.
Separation science
A surprisingly large part of chemistry has to do with isolating one component from a mixture. This may occur at any number of stages in a manufacturing process, including the very critical steps involved in removing toxic, odiferous, or otherwise undesirable by-products from a waste stream. But even in the research lab, a considerable amount of effort is often devoted to separating the desired substance from the many components of a reaction mixture, or in separating a component from a complex mixture (for example, a drug metabolite from a urine sample), prior to measuring the amount present.
|
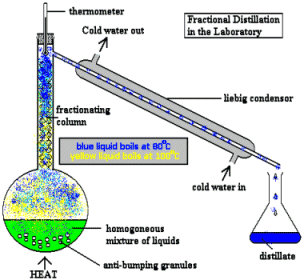
Distillation - separation of liquids having different boiling points. This ancient technique (believed to have originated with Arabic alchemists in 3500 BCE), is still one of the most widely employed operations both in the laboratory and in industrial processes such as oil refining. |
 |
Identification and assay
What do the following people have in common?
- A plant manager deciding on whether to accept a rail tank car of vinyl chloride for manufacture into plastic pipe
- An agricultural chemist who wants to know about the vitamin content of a new vegetable hybrid
- The manager of a city water-treatment plant who needs to make sure that the carbonate content of the water is maintained high enough to prevent corrosion, but low enough to prevent scale build-up
The answer is that all depend on analytical techniques — measurements of the nature or quantity ("assays") of some substance of interest, sometimes at very low concentrations. A large amount of research is devoted to finding more accurate and convenient means of making such measurements. Many of these involve sophisticated instruments; among the most widely used are the following:
|
Spectrophotometers that examine the ways that light of various wavelengths is absorbed, emitted, or altered by atomic and molecular species. |
 |
Materials, polymers, and nanotechnology
Materials science attempts to relate the physical properties and performance of engineering materials to their underlying chemical structure with the aim of developing improved materials for various applications.
|
Nanodevice chemistry — constructing molecular-scale assemblies for specific tasks such as computing, producing motions, etc. |
|
Biochemistry and Molecular biology
This field covers a wide range of studies ranging from fundamental studies on the chemistry of gene expression and enzyme-substrate interactions to drug design. Much of the activity in this area is directed to efforts in drug discovery.
|
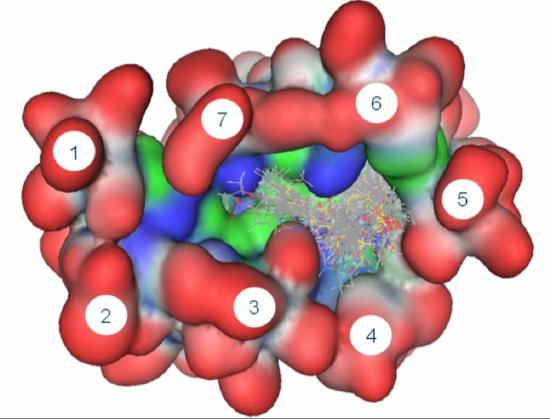
Drug design looks at interactions between enzymes and possible inhibitors. Computer-modeling is an essential tool in this work. |
 |
Synthesis
In its most general sense, this word refers to any reaction that leads to the formation of a particular molecule. It is both one of the oldest areas of chemistry and one of the most actively pursued. Some of the major threads are
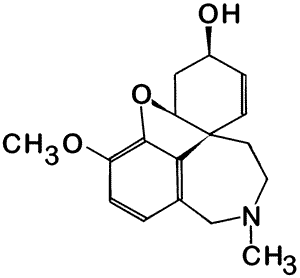
| New-molecule synthesis - Chemists are always challenged to come up with molecules containing novel features such as new shapes or unusual types of bonds. |
 |
|
Green chemistry - synthetic methods that focus on reducing or eliminating the use or release of toxic or non-biodegradable chemicals or byproducts. |
 |
Congratulations! You have just covered all of Chemistry, condensed into one quick and painless lesson— the world's shortest Chemistry course! Yes, we left out a lot of the details, the most important of which will take you a few months of happy discovery to pick up. But if you keep in mind the global hierarchy of composition/ structure, properties of substances, and change (equilibrium and dynamics) that we have developed in both macroscopic and microscopic views, you will find it much easier to assemble the details as you encounter them and to see where they fit into the bigger picture.
Contributors and Attributions
Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook