7.7: Equilibrium
- Page ID
- 96796
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- The Learning Objective of this Module is to establish that chemical reactions are reversible and to relate equilibrium constant to energy change.
Chemical Equilibrium
When first looking at chemical reactions, we typically say that reactants form products (proceed from left to right). Once products are present, however, they can go back to reactants. Reactions that go a significant amount in both directions are sometimes called reversible.
Reactants → Products and/or Reactants ← Products
Eventually, there is a balance between the two opposing processes, and no additional change occurs. The reversible chemical reaction is better represented at this point with a double arrow:
Reactants ⇌ Products
When the forward and reverse reactions are occurring at equal rate, their effects cancel each other out. A process at this point is considered to be at chemical equilibrium (or equilibrium). While the amounts of reactants and products seems to be unchanging, is important to note that the processes do not stop. They balance out each other so that there is no further net change; that is, chemical equilibrium is a dynamic equilibrium. Imagine 20 people in a room and every time one person leaves, another person enters; even though there is movement, the number of people stays constant.
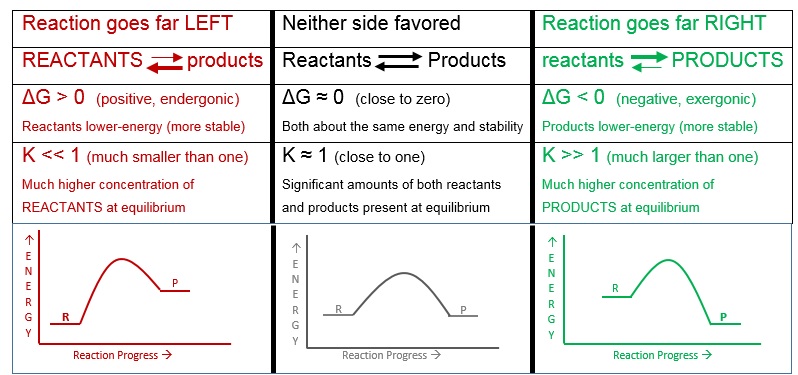
If the reaction is faster to the right, the concentration of products (on the right) will be larger at equilibrium. If the reaction is faster to the left, there will be a higher concentration of reactants (on the left) at equilibrium. If neither direction is faster, the concentrations will be roughly equal at equilibrium. The ratio of products to reactants at equilibrium is called the equilibrium constant, K.
The equilibrium constant expression always has products on top and reactants on bottom. The amounts of each are usually expressed as either concentrations, [brackets], or as pressures, P, if gases. (Note: We are simplifying this by assuming only one reactant and one product. The expression is typically more complicated.)
.jpg?revision=1&size=bestfit&width=552&height=74)
A K-value that is close to one will result in roughly equal amounts of reactants and products at equilibrium. Large K-values favor products and small K-values favor reactants. Thus equilibrium constants indicate how far a reaction will go to the right or left. This should sound familiar. Remember that energy, in particular Gibbs free energy (G), also indicates how far a reaction will go. While we will not do the calculations, K can be calculated from ΔG and temperature. Figure 7.7.1 below summarizes the relationship.
Figure 7.7.1 - The Relationship Between Reaction, Energy, and Equilibrium
Concept Review Exercise
-
What can be determined about the equilibrium constant and direction of a reaction if it has a negative Gibbs free energy change (exergonic)?
Answer
-
The reaction is likely to favor products and the equilibrium constant will be large.
Key Takeaway
- Many reactions are reversible and exist in equilibrium.
- The equilibrium constant is the ratio of the concentration of products over concentration of reactants.
Exercises
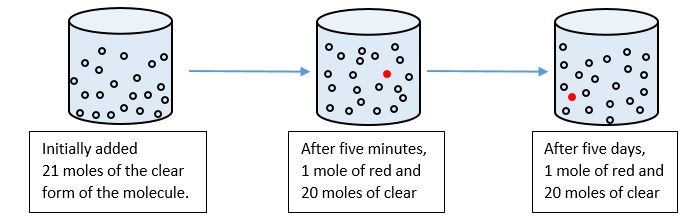
- A particular molecule converts between clear and red forms in an equilibrium reaction, clear ⇌ red. In the images below, each clear dot represents one mole of the molecules in clear form and each red colored dot represents one mole of the molecules in the red form (always in a constant one-liter volume). If the reaction takes place as shown below (starting with 21 moles clear, then ending with 20 moles clear and 1 mole red at equilibrium), what is the value of the equilibrium constant? Is this reaction endergonic or exergonic?
- What would exist in the container at equilibrium if 42 moles of the clear form of the molecule were added initially? (Same reaction and conditions as above.)
- What would exist in the container at equilibrium if 21 moles of the RED form of the molecule were added initially? (Same reaction and conditions as above.)
Answers
- K = [products] / [reactants] = [red] / [clear] = [1] / [20] = 0.05
A small K value (0.05 < 1) indicates that the reaction is endergonic (positive ΔG).
- At equilibrium, there would be 2 moles of the red form and 40 moles of the clear form (to keep the same ratio). K = [red] / [clear] = [2] / [40] = 0.05
- At equilibrium, there would be 1 moles of the red form and 20 moles of the clear form (to keep the same ratio). K = [red] / [clear] = [1] / [20] = 0.05

