7.6: Energetics and Kinetics
- Page ID
- 93079
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- The Learning Objective of this Module is to review how energy changes can effect the direction (how far) and rate (how fast) of chemical reactions.
Energetics (Thermodynamics)
In chemistry, thermodynamics (from the Greek roots for heat and force, power, or energy) is the field that studies the energetics of chemical reactions. There are several different energy-related quantities. Section 7.1 discussed that energy, work, and heat are inter-related and share the same units (e.g. calories or joules). Enthalpy, H, and Gibbs free energy, G, can also be expressed in those same energy units. The change in enthalpy is usually equal to the amount of heat gained or lost, so those two are often used interchangeably. Thermodynamics can tell us both if a reaction is likely to proceed and how far it is likely to go.
A system that is lower in energy is considered more stable. A ball at the top of a ramp is higher-energy (less stable), whereas that ball on the floor is lower-energy (more stable). A system will tend to move from higher to lower energy to become more stable --- the ball will spontaneously roll down the ramp.
The degree disorder or randomness, often called entropy, S, can also affect chemical reactions. A process is more likely to take place of everything becomes more disordered. For example, in a game of Yatzee, the odds of getting all five dice with the same number --- all ones, all twos, all threes, all fours, all fives, or all sixes --- in one roll is 6 out of 46 656 (very low). The odds that the roll will be less ordered (not five of a kind) will be the remaining 46 650 out of 46 656 (very high). There are just more ways to be random, so you are more likely to end up that way.
Gibbs free energy takes into account enthalpy (heat), temperature, and entropy (randomness). Because it takes into account more factors, the change is Gibbs free energy is the best for predicting which way a reaction will go and how far. A reaction will take place spontaneously from left to right (reactants to products) if the Gibbs free energy change is negative (going down in energy to become more stable). The more negative the change, the further the reaction will go. If the Gibbs free energy change is positive (going up in energy to become less stable), it will not take place spontaneously; instead it will go backward. Sometimes it is difficult to determine the change in Gibbs free energy, so we often use things like enthalpy change, or even the term “energy change” in a general sense instead.
A reaction energy diagram can summarize the energetics taking place. These diagrams show some type of energy on the vertical axis --- higher up is higher in energy. They show reaction progress, or time, on the horizontal axis --- further to the right means the reaction has gone further, or more time.
Figure 7.6.1 below shows a reaction in which the reactants are higher in energy and the products are lower in energy. If looking at energy (E) or enthalpy (H), the reaction would be described as “exothermic” because it would give off (lose or emit) heat. Reactions with negative changes in energy or enthalpy are more likely to be spontaneous. If examining Gibbs free energy, the reaction would be described instead as “exergonic” and would spontaneously proceed to the right (favors products).

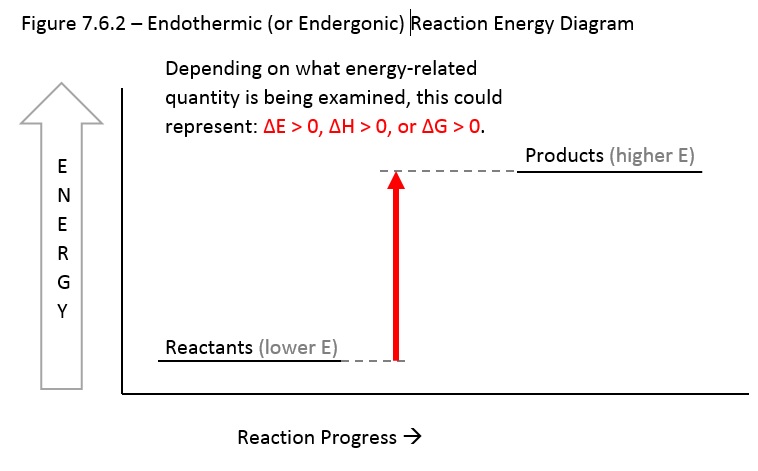
Figure 7.6.2 below shows a reaction in which the reactants are lower in energy and the product are higher in energy. If looking at energy (E) or enthalpy (H), the reaction would be described as “endothermic” because it would take in (gain or absorb) heat. Reactions with positive changes is energy or enthalpy are less likely to be spontaneous. If examining Gibbs free energy, the reaction would be described instead as “endergonic” and would not spontaneously proceed to the right; instead it would spontaneously proceed in reverse to the left (favors reactants).
Kinetics
The energetics (or thermodynamics) tell us if a reaction is likely to proceed and how far. Another important factor to consider is how FAST a reaction will go, also called KINETICS.
Two common forms of carbon are diamond and graphite. Diamond is less stable and, under normal conditions, is spontaneously being converted to the more stable graphite form. However, you do not need to rush out and sell your diamond jewelry. This reaction is terribly slow and will not take place in your lifetime. There is a barrier to getting a reaction started called the activation energy. Before the new bonds can form, some of the old bonds must be broken. (This is also why a spark is required to start a flame.) If the reaction energy barrier is larger, the reaction will be slower.
In Figure 7.6.3 below, the reaction energy diagram on the left has a larger activation energy barrier and would be slower. The smaller activation energy shown on the right would have a faster rate of reaction.

Activation energy also incorporates other factors that we are not discussing here. Nonetheless, it can help us understand other phenomena. Several things can increase the rate of a reaction. Here, we will look at three factors that can help reactants get over the activation energy barrier faster: temperature, concentration, and catalysts.
You likely know that food cooks faster at higher temperature. In general, reactions proceed at higher rate at higher temperature. Temperature is proportional to average kinetic energy, or how fast the reactants are moving. At higher temperatures, more of the reactants have enough energy to climb the activation energy barrier and thus can proceed to products faster.
Another way to increase the rate of a reaction is by increasing concentration of the reactants. Most reactions require the reactants to collide before they become products. If the concentration of reactants is higher, they are more crowded and thus collide more frequently. It was not mentioned in the previous paragraph but, at higher temperatures, faster-moving reactants also collide more frequently.
Catalysts are substances that speed up a reaction without being used up themselves. Because a catalyst is not consumed in a reaction, only a small amount is required. Catalysts make a reaction faster by lowering the activation energy barrier for the reaction. Instead of climbing the highest peak, a catalyst provide an easier pathway to the other side. If the two reaction energy diagrams shown previously in Figure 7.6.3 were the same reaction, the diagram on the left might represent the reaction without a catalyst. The diagram on the right might represent the same reaction, but in the presence of a catalyst (and with a lower energy of activation).
Example \(\PageIndex{1}\)
(A) Which of the two reaction energy diagrams shown below is more likely to proceed to products?
(B) Which of the two reaction energy diagrams shown would be the fastest?

SOLUTION
- The reaction, E --> F, is more likely to proceed to products. The products are more stable (exothermic or exergonic).
- The reaction, G --> H, would be faster. It has a smaller activation energy barrier.

Concept Review Exercise
- What word is used to describe a reaction with (a) positive enthalpy change or with (b) positive gibbs free energy change?
- What quantity determines the rate of reaction?
Answer
- (a) ΔH > 0 is endothermic; (b) ΔG > 0 is endergonic
- Activation energy, Ea
Key Takeaway
- Reactions are more likely to proceed spontaneously if the products are more stable (negative ΔE, ΔH, ΔG).
- The reaction rate will be faster if it has a lower activation energy barrier (Ea).

