2.3: Broadening Mechanisms
- Page ID
- 363053
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Spectrum lines are not infinitesimally narrow; they have a finite width. A graph of radiance or intensity per unit wavelength (or frequency) versus wavelength (or frequency) is the line profile. There are several causes of line broadening, some internal to the atom, others external, and each produces its characteristic profile. Some types of profile, for example, have a broad core and small wings; others have a narrow core and extensive, broad wings. Analysis of the exact shape of a line profile may give us information about the physical conditions, such as temperature and pressure, in a stellar atmosphere.
An absorption lineshape can represent the dynamics of the dipole or be broadened by energy relaxation, for instance through coupling to a continuum. However, there are numerous processes that can influence the lineshape. These can be separated by dynamic processes intrinsic to the molecular system, which is termed homogeneous broadening, and static effects known as inhomogeneous broadening, which can be considered an ensemble averaging effect.
Homogeneous Broadening
Several dynamical mechanisms can potentially contribute to damping and line-broadening. These intrinsically molecular processes, often referred to as homogeneous broadening, are commonly assigned a time scale \(T _ {2} = \Gamma^{- 1}\).
Population Relaxation
Atoms and molecules emit (or absorb) light when their electrons jump from one energy state to another. In the case of the H-alpha line, the transition involves the n=3 and n=2 states of hydrogen.
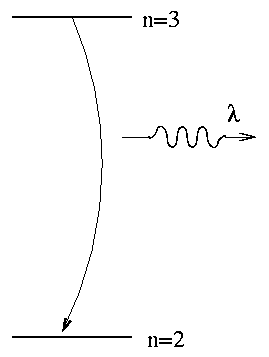
If we knew the energy difference exactly, then we would know the wavelength of the photon exactly. However, due to Heisenberg's Uncertainty Principle, we do NOT know the energies exactly. One way to look at is that atoms very quickly jump from an excited state to a lower state; that means that you don't have very long to measure the energy in the upper state. The limit on the time available to measure the excited state's energy places a limit on the precision of the energy measurement:
\[\Delta E \Delta t \simeq \hbar \nonumber \]
The lifetime of hydrogen atoms in their excited states is very short. For example, the lifetime of hydrogen in the \(n=2\) and \(n=3\) states is about \(10^{-8}\) seconds.
Population relaxation refers to decay in the coherence created by the light field as a result of the finite lifetime of the coupled states, and is often assigned a time scale \(T_1\). This can have contributions from radiative decay, such as spontaneous emission, or non-radiative processes such as relaxation as a result of coupling to a continuum.
\[\frac {1} {T _ {1}} = \frac {1} {\tau _ {r a d}} + \frac {1} {\tau _ {N R}} \label{11.61} \]
The observed population relaxation time depends on both the relaxation times of the upper and lower states (\(m\) and \(n\)) being coupled by the field:
\[1 / T _ {1} = w _ {m n} + w _ {n m}. \nonumber \]
When the energy splitting is high compared to \(k_BT\), only the downward rate contributes, which is why the rate is often written \(1/2T_1\).
Pure Dephasing (Collisional Broadening)
Pure dephasing is characterized by a time constant \(T_2^{*}\) that characterizes the randomization of phase within an ensemble as a result of molecular interactions. This is a dynamic effect in which memory of the phase of oscillation of a molecule is lost as a result of intermolecular interactions that randomize the phase. Examples include collisions in a dense gas, or fluctuations induced by a solvent. This process does not change the population of the states involved.
.png?revision=1)
Orientational Relaxation
Orientational relaxation (\(\tau_{or}\)) also leads to relaxation of the dipole correlation function and to line-broadening. Since the correlation function depends on the projection of the dipole onto a fixed axis in the laboratory frame, randomization of the initial dipole orientations is an ensemble averaged dephasing effect. In solution, this process is commonly treated as an orientational diffusion problem in which \(\tau_{or}\) is proportional to the diffusion constant.
If these homogeneous processes are independent, the rates for different processes contribute additively to the damping and line width:
\[\frac {1} {T _ {2}} = \frac {1} {T _ {1}} + \frac {1} {T _ {2}^{*}} + \frac {1} {\tau _ {o r}} \label{11.62} \]
Inhomogeneous Broadening
Absorption lineshapes can also be broadened by a static distribution of frequencies.
Doppler broadening
When thermal motion causes a particle to move towards the observer, the emitted radiation will be shifted to a higher frequency. Likewise, when the emitter moves away, the frequency will be lowered. For non-relativistic thermal velocities, the Doppler shift in frequency will be:
\[\displaystyle \nu=\nu_{0}\left(1+{\frac {v}{c}}\right), \label{eq10} \]
where \({\displaystyle \nu}\) is the observed frequency, \({\displaystyle \nu_{0}}\) is the rest frequency, \({\displaystyle v}\) is the velocity of the emitter towards the observer, and \({\displaystyle c}\) is the speed of light.
Since there is a distribution of speeds both toward and away from the observer in any volume element of the radiating body, the net effect will be to broaden the observed line. Propagating the Maxwell-Boltzmann Distribution law for velocities of a gas with the Doppler shifting (Equation \ref{eq10}) results in a line broadening (of a Gaussian distribution).
\[\Delta v_{\mathrm{FWHM}}=\sqrt{\frac{8 k T \ln 2}{m c^{2}}} v_{0} \nonumber \]
where FWHM is the full width at half maximum of the line.
Static Broadening
If molecules within the ensemble are influenced static environmental variations more than other processes, then the observed lineshape reports on the distribution of environments. This inhomogeneous broadening is a static ensemble averaging effect, which hides the dynamical content in the homogeneous linewidth. The origin of the inhomogeneous broadening can be molecular (for instance a distribution of defects in crystals) or macroscopic (i.e., an inhomogeneous magnetic field in NMR).
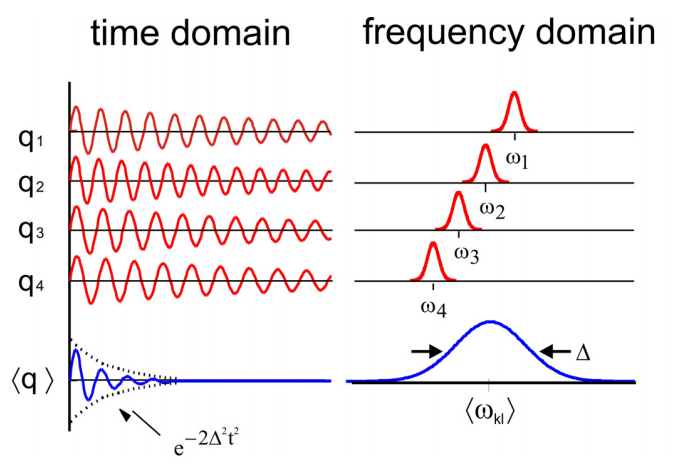
The inhomogeneous linewidth is dictated the width of the distribution \(\Delta\).
Total Linewidth
The total observed broadening of the absorption lineshape reflects the contribution of all of these effects. All of these effects can be present simultaneously in an absorption spectrum.

