8.2: Lab - Formation Constants and Complex Ions
- Page ID
- 354318
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
Goals:
- Understand what coordination complexes are
- Describe the formation of a coordination complex in terms of Lewis Acid-Base Theory
- Understand the method of continuous variations
- Calculate Kf from from spectroscpopic data
By the end of this lab, students should be able to:
- Design an experiment using Job Plot (the method of successive variations)
- Predict the formula for a complex ion using a Jobs plot
- Use a Job plot to calculate Kf.
Prior knowledge:
- Lewis Acids and Bases (section 16.7)
- Equilibrium Chemistry (section 15.4)
- Mechanisms (section 14.6)
Concurrent Reading:
- Equilibrium Involving Complex Ions (section 17.6)
Safety
- Emergency Preparedness
- Eye protection is mandatory in this lab, and you should not wear shorts or open toed shoes.
- Ferrous sulfate PubChem LCSS
- 1,10 Phenanthroline PubChem LCSS
- Minimize Risk
- label all containers
- Recognize Hazards
- All solutions should be considered harmful and care should be taken to avoid contact with your skin or other body tissues.
- In event of contact with reagents you should flush contacted area with water and notify instructor immediately.
- All waste is placed in the labeled container in the hood and will be recycled when the lab is over. Contact your instructor if the waste container is full, or about full.
Equipment and materials needed
| 0.005M Ferrous sulfate | 0.005M 1,10-Phenanthroline | 50 mL burette w/ringstand |
| 10-50 mL beakers | spectrometer | cuvettes |
Background
In this lab we are going to run a Jobs plot to determine the formation constant for the formation of a coordination complex of iron(II) and 1,10 phenanthroline. A Jobs plot is similiar to a titration. In acid/base titrations the pH is measured as a function of the amount of titrant (strong acid or base) that is added to the analyte (base or acid). The titration starts with pure analyte, then a buffer is formed while the analyte is the excess reagent and only part of it has been converted to its salt. At the equivalence point the two reagents are in stoichiometric proportions and upon further addition of titrant the analyte becomes the limiting reagent and the pH is dictated by the excess titrant. In a pH titration the pH is changing for two reasons, (1) neutralization and (2) dilution. In a Jobs plot the total volume is kept constant and so the reactant and product concentrations are not influenced by dilution. Unlike a titration where you continually add one reactant to another and measure the effect, in a Job's plot each measurement is a unique solution where the mole fraction of reactants are varied. If a Job's plot had 20 measurements you would need to make 20 different solutions, where each solution represented different mole fraction ratios. Another name for the Job's Plot is the method of continuous variations and in this lab the Job's plot technique will be be applied to the formation of a coordination complex ion.
Coordination Complexes
Many metal ions have vacant d orbitals that allow them to function as Lewis Acids (electron acceptors) that can react with molecules (or ions) containing lone pairs of electrons, the Lewis bases (electron donors). These reactions can result in the formation of a coordination complex (sections 16.7: & 17.6), which are often highly colored. In this type of reaction the Lewis base is called the Ligand (L) and may be neutral (NH3, H2O,...) or charged (Cl-, NO2-, ...). The sum of the charges of the metal cation and all the ligands bonded to it gives the charge of the coordination complex, which may result in a neutral, cationic (positive) or anionic (negative) complex. Charged coordination complexes are known as complex ions and are often soluble and highly colored. A complex ion consists of a central metal atom and a specific number of ligands covalently bonded to it, which determines its chemical formula. Water is a typical Lewis base and common aqueous metal ions like Fe+2, Fe+3, Cu+2 and Al+3 form complex ions with water, that is, they form covalent bonds with the water, where both electrons come from the water.
Coordination complexes will be studied in more detail when we get to chapter 20 and for this lab it suffices to know that a complex ion can form from the Lewis Acid-Base reaction between a metal cation and a ligand (sections 16.7: & 17.6).

A ligand may be monatomic (Cl-) or polyatomic (NH3). For polyatomic ligands the donor atom of the ligand is the atom that has the lone pair of electrons that form the coordinate covalent bond with the metal ion (oxygen is the donor atom of water in figure \(\PageIndex{1}\)). A polyatomic ligand may have more than one donor atom and thus form more than one coordinate covalent bond. In figure \(\PageIndex{2}\) there are two ligands where nitrogen functions as the donor atoms. A ligand with one donor atom is classified as a monodentate ligand, a ligand with two donor atoms is a bidentate ligand, and there are common polydentate ligands with up to 6 donor atoms.
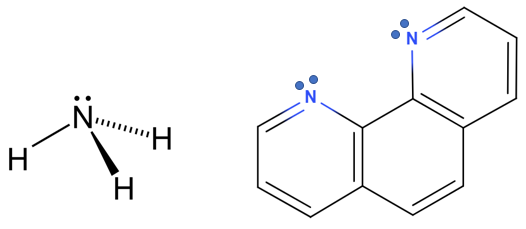
The resulting coordination complex has a specific number of coordinate covalent bonds, which is the coordination number of the complex. Figure \(\PageIndex{3}\) shows generic coordination complexes with various numbers of monodentate ligands and one bidentate ligand. The coordination number is only equal to the number of ligands if the ligands are monodentate, and in the case of the bidentate ligand with a formula ML3 the coordination number (CN) is 6. Typical coordination numbers vary from 2 to 6 and the complexes have geometries similar to the VSEPR geometries we learned about in the first semester of the class.

Figure \(\PageIndex{3}\): Formula and coordination number for different coordination complexes. Note the complex ion on the right involves a bidentate ligand where oxygen is the donor atom and the formula ML3 results in a coordination number of 6.
In this experiment we will be creating a highly colored complex ion between ferrous iron (Fe+2) and a neutral bidentate ligand (figure \(\PageIndex{2}\)). We will then determine the formula for the complex ion and its formation constant. The generic equation for the formation of a complex with "b" ligands has the following form and formation constant (KF).
\[M + bL \rightleftharpoons ML_b \;\; \;\;\left ( K_f=\frac{[ML_b]}{[M][L]^b} \right )\]
We will use absorbance spectroscopy and make measurements at a wavelength where the metal and the ligand are both colorless, but the complex MLb is highly colored, and thus monitor the formation of the complex by the intensity of its color. The technique we will used is called the Job's Plot, or the method of continuous variations.
Job's Plot
A Job's plot is also know as the method of continous variations, where you measure the concentration of the product after varying the mole fractions of the reactants, and then plot the concentration of product as the dependent variable, with the mole fractions as the independent variable. Since there are only two species there are two scales on the x axis, one for the mole fraction ligand and the other for the mole fraction of the metal, and they are related by the equation XM + XL = 1.
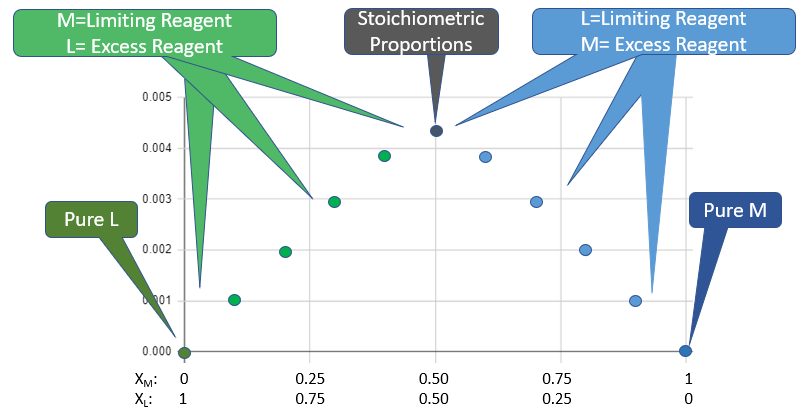 Figure \(\PageIndex{4}\): Regions of Job Plot. Note, the scale on the X-axis shows the mole fractions of metal (XM) and Ligand (XL) and since these are related to each other (XM + XL=1), with the fraction varying from pure ligand on the left to pure metal on the right. The peak represents when they are mixed in stoichiometric proportions. (Belford)
Figure \(\PageIndex{4}\): Regions of Job Plot. Note, the scale on the X-axis shows the mole fractions of metal (XM) and Ligand (XL) and since these are related to each other (XM + XL=1), with the fraction varying from pure ligand on the left to pure metal on the right. The peak represents when they are mixed in stoichiometric proportions. (Belford)The peak of jobs plot identifies the ratio of ligand to metal at stoichiometric proportions and thus allows one to determine the formula of the coordination complex. One can also look at the ratios of the slopes to in the regions away from stoichiometric proportions to determine the formula and you will be asked to devise this technique in your report. When doing so it is important to realize that you are not measuring the reactant concentrations but the product concentration, which is plotted as a function of the reactant mole fraction.
Comparing Job's Plot to a Titration
Both titrations and Job's plots start with a pure reactant and then measure some effect from adding a second substance that reacts with the first. In an acid base reaction this is the hydronioum ion concentration (pH). It should be emphasized that acid-base titrations are only one type of titration, and other types of titrations like redox titrations are common. In a titration there is only one solution and you make measurements as you successively add the titrant. In the Job's plot each measurement is a different solution. In the titration the concentration of the initial reactant (analyte) is being reduced by two processes, reaction with the titrant and dilution, as the total volume of the solution increases as you add titrant. In the Job's plot you keep the total volume constant while varying the proportions of the different reagents mixed, so in a Job's plot you do not have an analyte and titrant, but start with a solution that is pure reactant "A" and end with one that is pure reactant "B". In both titrations and Job's plots you need a way to measure the progress of the reaction. In pH titrations this can be down with an indicator or pH meter. In a Job's plot this is often done with spectroscopy because the complexes are often highly colored, while the metal solutions and ligands are often colorless.
Beer's Law
Coordination complexes can be highly colored and we will use a spectrometer to measure the concentration of the coordination complex. According to Beer's Law the concentration is proportional to the absorbance. \[A=\epsilon bc\] where \(\epsilon\) = extinction coefficient, b=path length and c=concentration (experiment 3.2b). You need to review the use of spectrometers (section 0.3 of the general information) and realize that for the spectrometer we are using, you can only trust Beer's law over the absorbance range of 0.05 to 1.
Experimental Procedures:
- Fill 50 mL burette with 0.005 M ferrous iron.
- Fill 50 mL burette with 0.005 M unknown ligand.
- Label fifteen 50 mL beakers with the mole fractions of iron as in the data sheet.
- Transfer to labeled beakers the proper amount of solution. Each beaker should contain 10 mL of total solution.
- Wait at least 20 minutes for the reaction to complete.
- Choose wavelength for absorbance measurements.
- Zero out the spectrometer with a blank solution of water.
- Take a spectra of the darkest solution.
- Choose a wavelength where the darkest solution approaches 1.
- Write this wavelength down on the data sheet.
- Record the absorbance of each solution in data sheet at the wavelength chosen in step 6.
- Double check that the reaction is over by checking the solutions that represent stoichiometric proportions of the hypothetical complexes to see if the absorbance has increased. Once all solutions maintain a constant value you can work up the data.
Data Analysis:
Overview
Figure \(\PageIndex{8}\) shows the features of the Job's plot analysis you will develop with your Google Workbook. For a bidentate ligand we expect three possible formulas; ML3 (red line at xm=0.25), ML2 (purple line at xm= 0.33) and ML (green line at xm= 0.50). You will plot three series of data in your sheet. Series A is all the data. Series B (in red) is the data where the ligand is in excess and drives the metal to be completely consumed. Series C (in yellow) is where the metal is in excess and drives the ligand to be completely consumed. If there was no equilibria and all the reactants convert to products these two trend lines would cross when the reagents are in stoichiometric proportions. From the graph this occurs closest to the mole fractions associated with ML3., and so we will consider this to occur at mx = 0.25. It is clear that as the proportions approach stoichiometric proportions the real curve (blue dots) deviate from the straight line functions where a reagent was in excess and this is due to the fact the an equilibria exists between coordination complex and the metal and ligands.
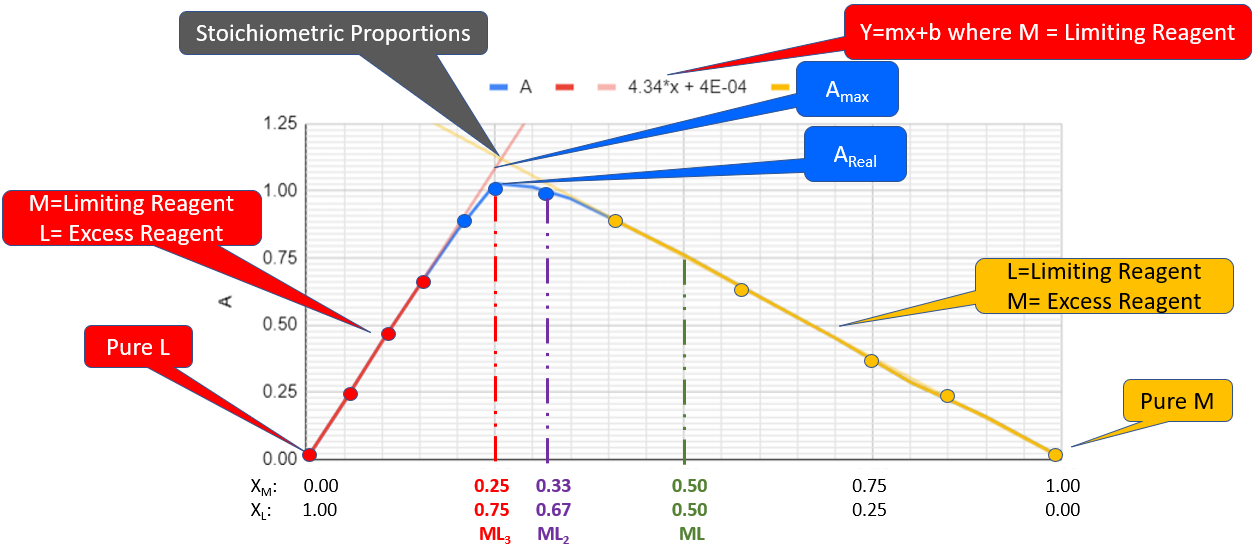 Figure \(\PageIndex{8}\): Jobs plot within the Google Workbook. Note there are three concentrations where the reactants could be in stoichiometric proportions. ML3 (red at Xm=0.25), ML2 (purple at Xm=0.33) and ML (green at Xm=0.50). In this graph the trend lines from the regions where one reagent was in excess cross at around 0.27, so we will consider the formula to be ML3 and the complex forms at xm=0.25 and cL=0.75 (Copyright; Bob Belford & Liliane Poirot CC-BY)
Figure \(\PageIndex{8}\): Jobs plot within the Google Workbook. Note there are three concentrations where the reactants could be in stoichiometric proportions. ML3 (red at Xm=0.25), ML2 (purple at Xm=0.33) and ML (green at Xm=0.50). In this graph the trend lines from the regions where one reagent was in excess cross at around 0.27, so we will consider the formula to be ML3 and the complex forms at xm=0.25 and cL=0.75 (Copyright; Bob Belford & Liliane Poirot CC-BY)Areal
Areal is the absorbance of the solution taken from the data sheet when they are in stoichiometric proportions. for figure \(\PageIndex{8}\) this was 1.027 (the data is not shown, but use the value of your data sheet when they are in stoichiometric proportions)
Amax
Amax is the value of the absorbance if the theoretical yield was 100%. This can be calculated by using the equation for the straight line in the excess ligand region and calculating the value of A at xm=0.25. That is, when there was excess ligand it essentially used up all the limiting reagent as the system achieved equilibrium.
\[A_{max}=4.34(0.25) + 0.0004 = 1.085\]
Cmax
Cmax is based on 100% theoretical yield when the reactants are in stoichiometric proportions (where the two trend lines intersect). For figure \(\PageIndex{9}\) this occurs as xm= 0.25 and if the initial ferrous iron concentrtation was 0.005M, the complex ion concentration [FeL3+2] is:
\[0.25(.005M)= 0.00125M\]
Creal
To calculate Kf we need to know the concentration of the coordination complex and this can be done by applying the two state approach to Beer's Law (A=\(\epsilon bc\)) and simplifying. It should be noted the C represents the complex ion concentration ([MLb]+2 for a complex with b ligands.
\[\frac{A_1}{A_2} =\frac{\epsilon bC_1}{\epsilon bC_2} \\ \; \\ so \\ \; \\ \frac{A_1}{A_2} =\frac{C_1}{C_2}\]
So if state "1" is the real concentration and state "2" is the hypothetical "max" concentration if there was 100% theoretical yield, we get
\[C_{real}=C_{max}\frac{A_{real}}{A_{max}}\]
Based on the above data, \[C_{real}=0.00125M\left ( \frac{1.027}{1.085} \right )=0.00118M\]
Calculating Kf
Figure \(\PageIndex{9}\) represents the formation of the complex ML because the peak is when the mole fraction of M and L are both 0.5. If we look at the complex MLb where "b" is the number of ligands we have three possible equilbrium constants and the following generic RICE diagram.
\[M + L \rightleftharpoons ML \;\;\left ( K_f=\frac{[ML]}{[M][L]} \right ) \\ \; \\ M + 2L \rightleftharpoons ML_2 \;\; \;\;\left ( K_f=\frac{[ML_2]}{[M][L]^2} \right ) \\ \;\\ M + 3L \rightleftharpoons ML_3 \;\;\;\;\left ( K_f=\frac{[ML_3]}{[M][L]^3} \right )\]
| Reactants | M | + | bL | ⇌ | MLb |
|---|---|---|---|---|---|
| Initial | [M]Initial | [L]Initial | 0 | ||
| Change | -x | -bx | +x | ||
| Equilibrium | [M]Eq=[M]Initial-x | [L]Eq=[L]Initial-bx | [MLb]Eq=x |
Noting CREAL is MLb because it is the complex ion that is absorbing the light. So we know know the extent of reaction (x), and can solve for the equilibrium concentration of all species using the Kf expression appropriate to the complex ion being formed (b=1, 2 or 3).
\[K=\frac{x}{(M_i-x)(L_i-bx)^b}\]
For the above example where xm=0.25 we have the formula of ML3 and when at stoichiometric proportions ML3= Creal=0.00118M
| Reactants | M | + | bL | ⇌ | MLb |
|---|---|---|---|---|---|
| Initial | .25(.005)=[0.00125] | 0.75(.005)=[.00375] | 0 | ||
| Change | -0.00118 | -3(0.00118) | +0.00118 | ||
| Equilibrium | 0.00007 | 0.00021 | 0.00118 |
\[K_f=\frac{0.00118}{0.00007\left ( 0.00021 \right )^3}=1.82x10^{12}\]
Cover Page
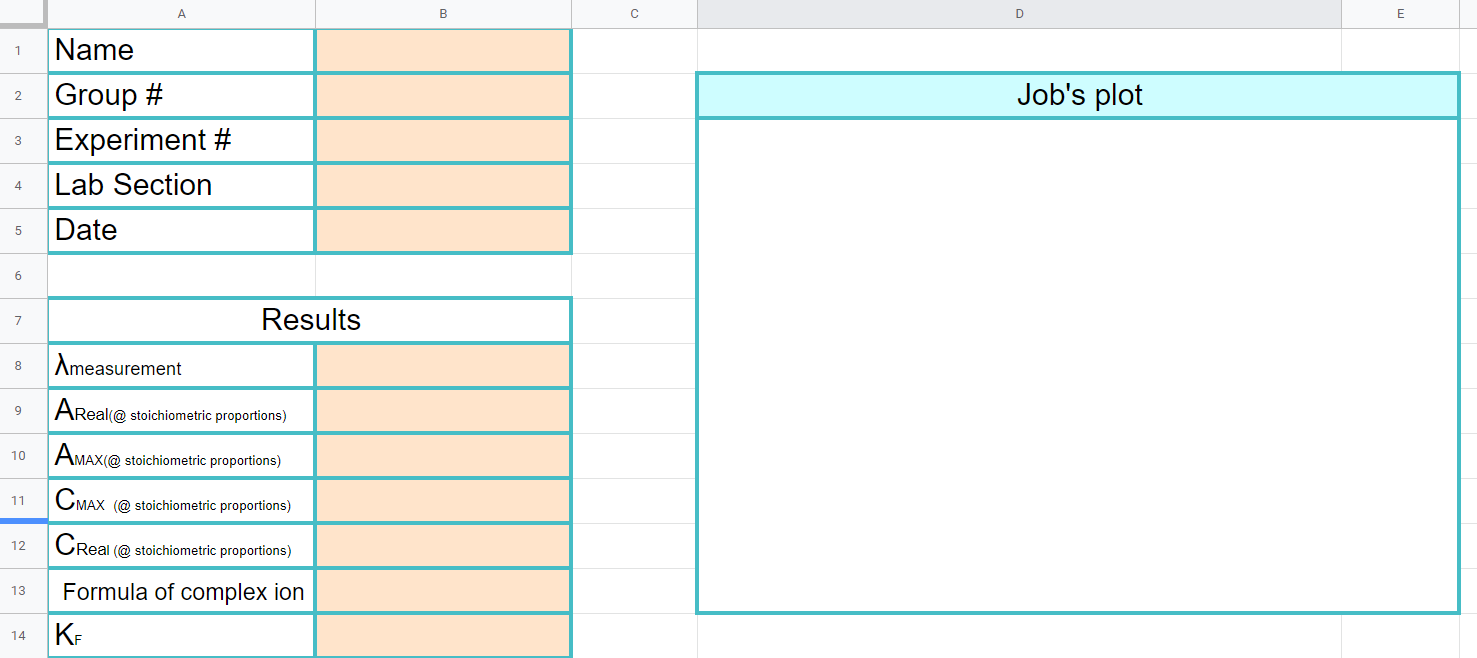
Figure \(\PageIndex{9}\): Note Cmax is the concentration of the complex ion based on the theoretical yield and Creal is the actual concentration of the complex ion due to an equilibria between reactants and products. (Copyright; Bob Belford & Liliane Poirot CC.0)
Note, for the formula use ML, ML2 or ML3. (you can make the font size of the numbers smaller so it looks like a subscript)
Data Sheet
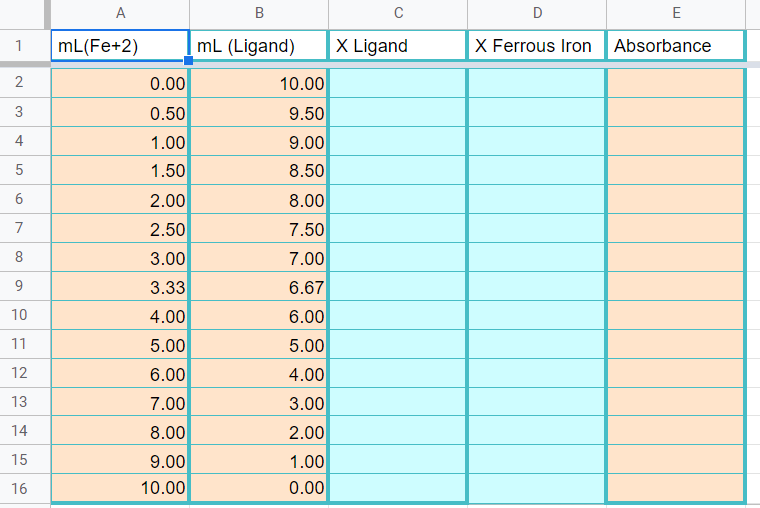 Figure \(\PageIndex{10}\): Copy the data from the data sheet handout to this page in your workbook. Be sure to copy the wavelength you measured the absorbance at on the title page (Copyright; Bob Belford & Liliane Poirot, CC0)
Figure \(\PageIndex{10}\): Copy the data from the data sheet handout to this page in your workbook. Be sure to copy the wavelength you measured the absorbance at on the title page (Copyright; Bob Belford & Liliane Poirot, CC0)
Job's Plot
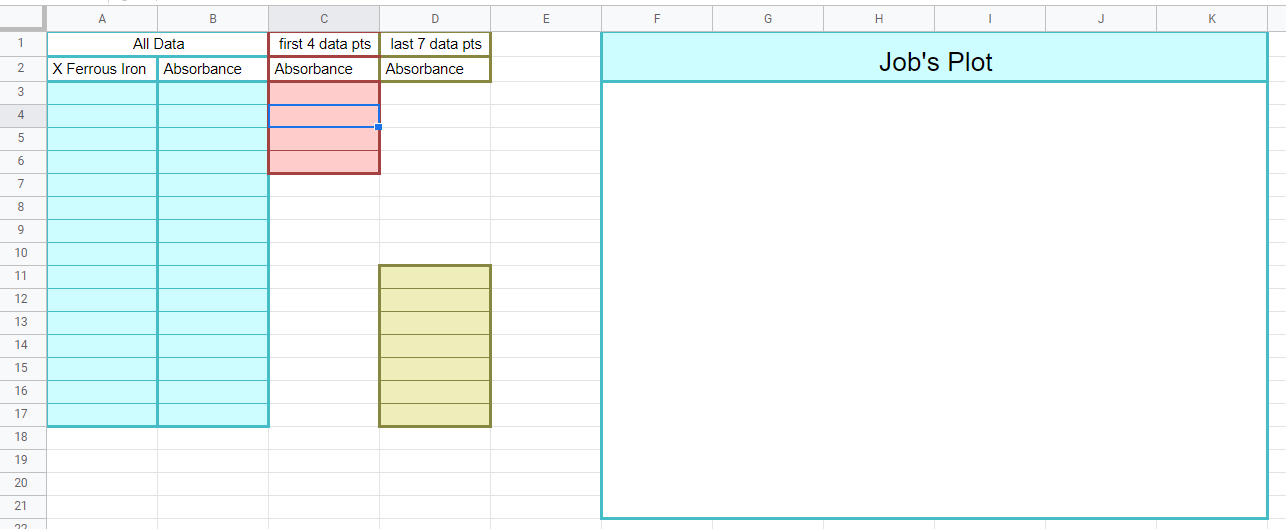 Figure \(\PageIndex{11}\): When completed this graph will have three series of data. The first is a scatter plot of all the data. The second is a trend line with equation of the first 4 datapoints (column C) and the last is the trendline of the last 7 data points (column D). Where the trendlines intersect will be used to determine the stoichiometric proportions of the metal and ligand as they form the complex ion (Copyright; Bob Belford & Liliane Poirot CC.0)
Figure \(\PageIndex{11}\): When completed this graph will have three series of data. The first is a scatter plot of all the data. The second is a trend line with equation of the first 4 datapoints (column C) and the last is the trendline of the last 7 data points (column D). Where the trendlines intersect will be used to determine the stoichiometric proportions of the metal and ligand as they form the complex ion (Copyright; Bob Belford & Liliane Poirot CC.0)
- Paste data into columns A and B using ctrl+shift+v
- Copy first 4 absorbance data points
The following YouTube provides instructions for creating the Job's plot.
Rice Table
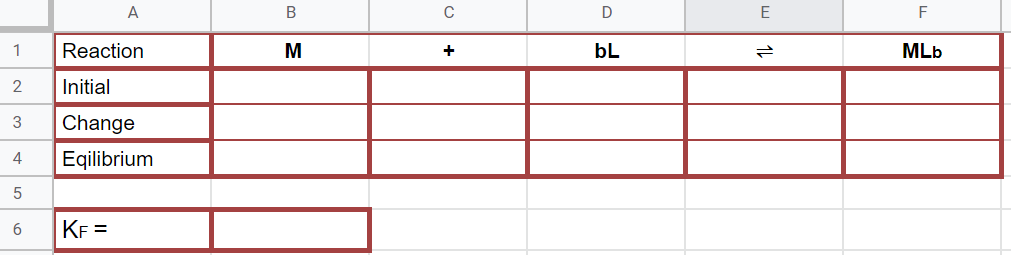 Figure \(\PageIndex{12}\): Rice diagram for calculating the formation constant. (Copyright; Bob Belford & Liliane Poirot CC0.0)
Figure \(\PageIndex{12}\): Rice diagram for calculating the formation constant. (Copyright; Bob Belford & Liliane Poirot CC0.0)The data for the RICE table represents the reactants when they are mixed in stoichiometric proportions. The initial concentrations of the metal and ligand can be calculated from the mole fractions and the concentrations of the stock reagents mixed. The Equilibrium concentration [MLb] is Creal.
Note
If there are more than one ligand there would be intermediates formed through a mechanism involving bimolecular collisions with the ligands and metal ions, or ligands and the intermediates. That is, there is a stepwise formation of the complex ion
\[M + L \rightleftharpoons ML \;\;\;(fast) \\ \; \\ ML + L \rightleftharpoons ML_2 \;\;\;(slower) \\ \;\\ ML_2 + L \rightleftharpoons ML_3 \;\;\;(slowest)\]
When the metal is the limiting reagent and there is a lot of excess ligand (high XL or low XM), the theoretical yield based on the complete consumption of the limiting reagent (M) is achieved because of there is so little M that it all gets used up. Due to the excess [L] all of the equilibria are moved to the right and the final product is the major product. When the ligand is the limiting reagent it is also consumed, but now there is a paucity of L and so many of the intermediates can exist in equilibrium. In fact at very low ligand mole fractions the intermediates are often the dominant product. That is why in obtaining AMAX we extrapolate from the side where metal is the limiting reagent, and not where L is. If there were no intermediates (like in M + L ->ML) one could extrapolate back from the side of low ligand too, and where they meet would be the ratio of stoichiometric proportions (as in figure \(\PageIndex{9}\))
It should also be noted that the rates of the steps do not define the equilibrium constants, and that the formation constant of the final complex ion is often the largest.

