12.3: Bonding in Metals and Semiconductors
- Page ID
- 43271
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)To date we have avoided metallic bonds, and due to time, this course will be very weak on them, and semiconductors.
Characteristics of Metals
- low ionization energies (can easily lose electrons and form cations)
- low electronegativities (tend to have partial positive charge in polar bonds - are electropositive)
- lustrous (shiny)
- malleable - can be beat into thin sheets (their bonds are flexible and not rigid with a fixed geometry)
- ductile - can be drawn into wires.
- thermal and electrical conductors
Electron Sea Model
Figure 11.4.a. Pictorial representation of electron sea model.
Atomic cores (nuclei and core electrons) form the "spheres" while valence electrons become attracted to multiple nuclei, and are free to move about the bulk of the electron. This is a qualitative description but there is value to it.
Video \(\PageIndex{1}\): 0:26 Minute animation on electron sea model uploaded by Chemsurvival (https://youtu.be/V5tj-xADB1c)
Molecular Orbital (MO) and Band Theory
In general chemistry 1 we covered molecular orbital theory and saw how two orbitals could overlap to form a bonding orbital and an antibonding orbital. This was for simple diatomic molecules, isolated in space and Band Theory expands this to systems where many orbitals and electrons from multiple atoms mix. If you think about it, this is the fundamental premise of the Electron Sea Model, in that there is a mixing of the valence orbitals of multiple orbitals. And when we say multiple, we mean huge numbers (1023).
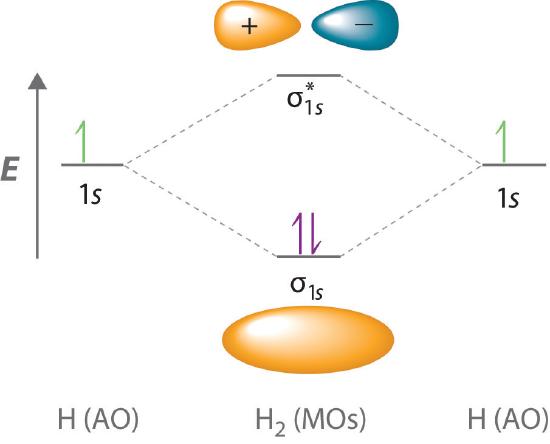
Figure 11.4.b. MO electron configuration for hydrogen showing bonding and antibonding orbitals
We then expanded this concept to the second period of the period table, and were able to explain phenomena like the diamagnetic properties of oxygen.
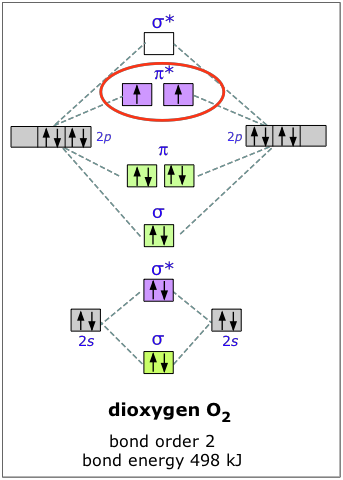
Figure 11.4.c. MO diagram for oxygen, showing how the 2s and three 2p atomic orbitals of 2 oxygen atoms create 8 molecular orbitals.
There are several postulate of MO theory that we should review
- The number of Molecular Orbitals equals the number of Atomic Orbitals mixed.
- Each orbital has a discrete energy
- Orbitals need to be of similar energy to mix,
- Bonding Orbital result when orbital wave functions complement each other
- Antibonding orbitals repulse when orbital wave functions cancel each other
When the number of orbitals becomes huge, the energies of the mixed orbitals merge and you effectively a blurring of the energy levels, and a band of available energy states.
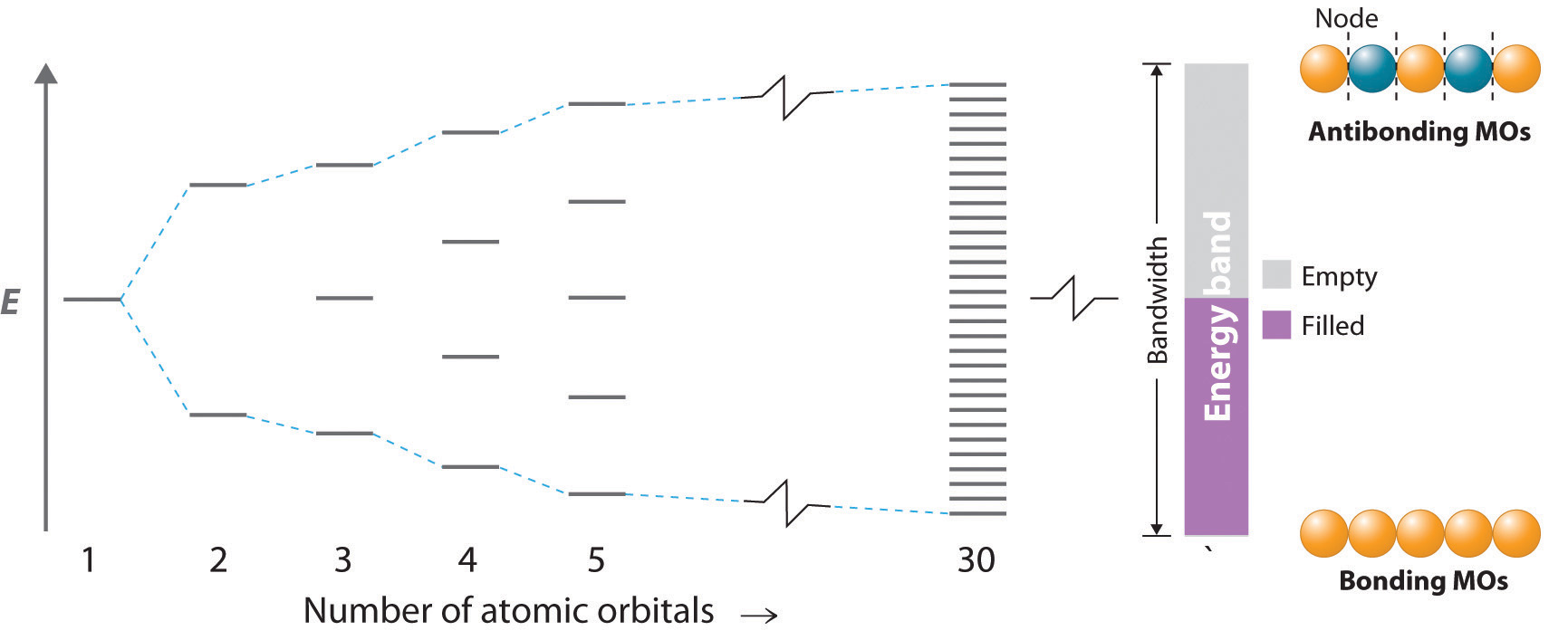
Metallic bonds involve electrons delocalized over multiple nuclei and so the number of atomic orbitals that combine to form molecular orbitals is so huge that the energy gap between them becomes negligible, and they in effect form bands. But there are two types of molecular orbirtals, bonding and antibonding. Electrons in the bonding orbitals form the valence band, and electrons in the antibonding are not localized on a specific nuclei and form the conductance band.
Conductors, Insulators and Semiconductors
We live in a world full of electronics and many of the students of this class are engineering students who need to know the basics behind the devices they will work with. Although during our exams this class will not go into band gap theory, it is worth mentioning.
A. Conductors
Metals are conductors. There is no band gap between their valence and conduction bands, since they overlap. There is a continuous availability of electrons in these closely spaced orbitals.
B. Insulators
In insulators, the band gap between the valence band and the conduction band is so large that electrons cannot make the energy jump from the valence band to the conduction band, and thus they stay localized to the nuclei they are associated with.
C. Semiconductors
Semiconductors have a small energy gap between the valence band and the conduction band. Electrons can make the jump up to the conduction band, but not with the same ease as they do in conductors.
Video\(\PageIndex{2}\) 6:31 min Youtube introducing introducing band theory as applied to conductivity and semiconductors (https://youtu.be/5zz6LlDVRl0)


