12.4: Network and Amorphous Solids
- Page ID
- 43272
Introduction
We have described solids in terms of metals and ionic compounds with crystalline structures. Atoms and molecules can also form extended networks of covalent bonds. There are two basic types of covalent network solids, either atomic networks or molecular networks.
- Covalent Network Solids
- Atomic Network Solids
- Molecular Network Solids
- Amorphous Solids
In the above
Atomic Network Solids
A molecule represents an specific chemical species of nuclei connected together with covalent bonds and a defined chemical formula. In contrast, the chemical formula of an ionic compound, which does not describe the smallest entity, but the ratio of cations and anions that results in a neutral structure. Yet there is another type of covalent compound which extends like the lattice of an ionic lattice, and that is the covalent network solid.
Video\(\PageIndex{1}\): Youtube
Diamond -
Diamond is made of covalent carbon-carbon bonds extending in three dimensions. The geometry is tetrahedral resulting from sp3 hybrid carbons and there is no molecular formula, as this results in a 3D extended network based on the hybridization.
Figure\(\PageIndex{1}\): Figure showing sp3 hybridized carbon in diamond atomic network
Graphite
Graphite is sp2 hybridized carbon forming planar sheets
Figure\(\PageIndex{2}\): Atomic network of carbon in graphite.
Exercise \(\PageIndex{1}\)
Allotropes are different forms of the same element. Diamonds and graphite are allotropes of carbon. Do you know another allotrope of carbon? The later of a specific molecular formula (C60) while diamond and graphite form extended networks of covalent bonds.
- Answer
-
Buckminsterfullerene is an allotrope of carbon. It is not a network solid as it has a formula, (C60).
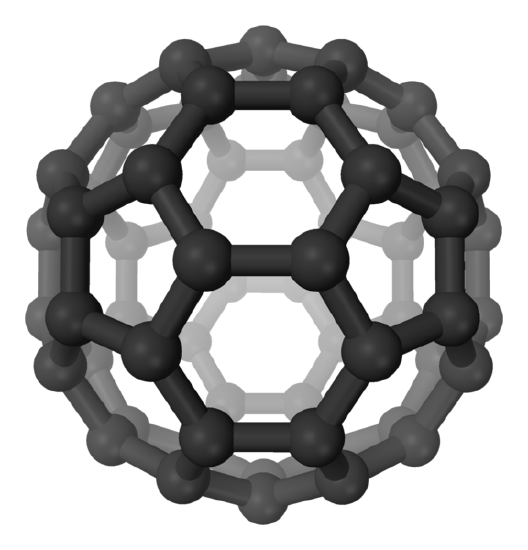
Molecular Network Solids
The difference between an atomic network and a molecular network is the molecular network consists of extended molecular units. Sand and ice are examples of molecular network solids

Figure\(\PageIndex{3}\): A major component of sand is silica, which has the approximate formula of SiO2, with this basic unit stretching in 3 dimensions as a molecular extended network. In reality the structure is variable, with hydrogen also being involved. (Image borrowed from Chris Schaller)
Amorphous Solid
Amorphous solids are non-crystalline and do not have repeating structural order. The word comes from greek "a" without and "morphe" (form). Glass is a type of amorphous solid, in fact there are arguments as to if glass is a solid or a liquid.

Figure\(\PageIndex{4}\): Desert glass, uploaded from wikicommons
Test Yourself
Query \(\PageIndex{1}\)
Contributors
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
Images credits
- Chris Schaller, WIkipedia,
- H. Raab, Wikicommons
- modified anonymous


