7.5: Atomic Properties and Periodic Trends
- Page ID
- 158452
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Define radius, ionization energy, electron affinity, electronegativity, metallic character, and oxidation potential for atoms.
- Predict how the atomic radius, ionization energy, electron affinity, electronegativity, metallic character, and oxidation potential various for atoms across period and in a group
- Justify the reason for such trends using effective nuclear charge
The elements in the periodic table are arranged in order of increasing atomic number. All of these elements display several other trends and we can use the periodic law and table formation to predict their chemical, physical, and atomic properties. Understanding these trends is done by analyzing the elements electron configuration; all elements prefer an octet formation and will gain or lose electrons to form that stable configuration.
Atomic Radius
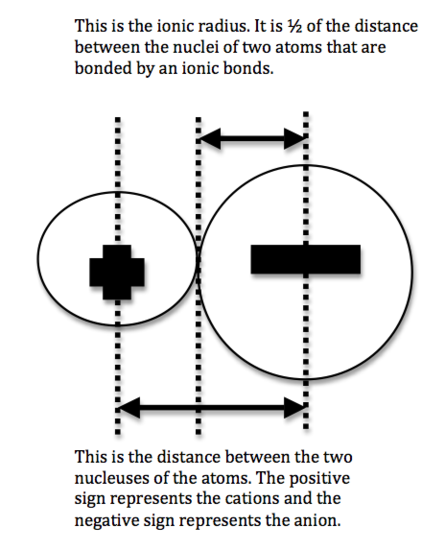
We can never determine the atomic radius of an atom because there is never a zero probability of finding an electron, and thus never a distinct boundary to the atom. All that we can measure is the distance between two nuclei (internuclear distance). A covalent radius is one-half the distance between the nuclei of two identical atoms. An ionic radius is one-half the distance between the nuclei of two ions in an ionic bond. The distance must be apportioned for the smaller cation and larger anion. A metallic radius is one-half the distance between the nuclei of two adjacent atoms in a crystalline structure. The noble gases are left out of the trends in atomic radii because there is great debate over the experimental values of their atomic radii. The SI units for measuring atomic radii are the nanometer (nm) and the picometer (pm). 1 nm = 1 X 10-9 m; 1 pm = 1 X 10-12 m.
Figure 1:Covalent Radii Figure 2: Ionic Radii Figure 3: Metallic Radii
To explain this trend, the concept of screening and penetration must be understood. Penetration is commonly known as the distance that an electron is from the nucleus. Screening is defined as the concept of the inner electrons blocking the outer electrons from the nuclear charge. Within this concept we assume that there is no screening between the outer electrons and that the inner electrons shield the outer electrons from the total positive charge of the nucleus. In order to comprehend the extent of screening and penetration within an atom, scientists came up with the effective nuclear charge, \(Z_{eff}\). The equation for calculating the effective nuclear charge is shown below.
\[Z_{eff}= Z - S\]
In the equation S represents the number of inner electrons that screen the outer electrons. Students can easily find S by using the atomic number of the noble gas that is one period above the element. For example, the S we would use for Chlorine would be 10 (the atomic number of Neon). Z is the total number of electrons in the atom. Since we know that a neutral atom has an identical number of protons and electrons, we can use the atomic number to define Z. For example, Chlorine would have a Z value of 17 (the atomic number of Chlorine). Continuing to use Chlorine as an example, the 10 inner electrons (S) would screen out the positive charge of ten protons. Therefore there would be and effective nuclear charge of 17-10 or +7. The effective nuclear charge shows that the nucleus is pulling the outer electrons with a +7 charge and therefore the outer electrons are pulled closer to the nucleus and the atomic radii is smaller. In summary, the greater the nuclear charge, the greater pull the nucleus has on the outer electrons and the smaller the atomic radii. In contrast, the smaller nuclear charge, the lesser pull the nucleus has on the outer electrons, and the larger atomic radii. Additionally, as the atomic number increases, the effective nuclear charge also increases. Figure 3 depicts the effect that the effective nuclear charge has on atomic radii.
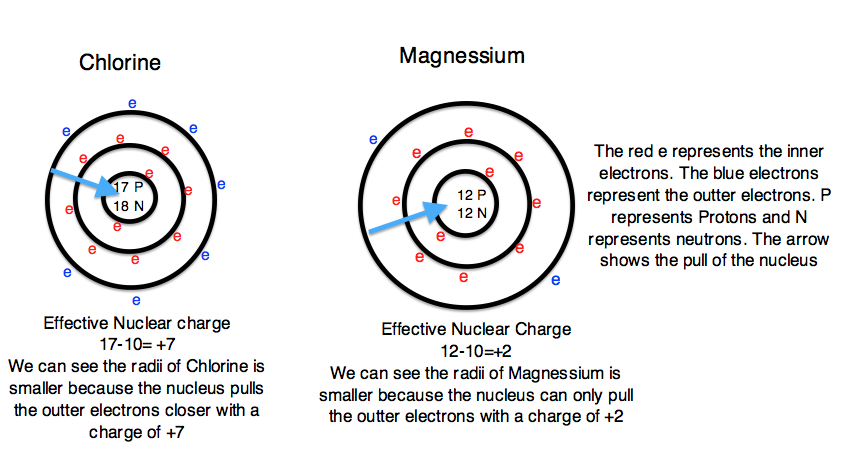
Figure 3: Courtesy of Jessica Thornton (UCD)
Now we are ready to describe the atomic radius trend in the periodic table. The atomic number increases moving left to right across a period and subsequently so does the effective nuclear charge. Therefore, moving left to right across a period the nucleus has a greater pull on the outer electrons and the atomic radii decreases. Moving down a group in the periodic table, the number of filled electron shells increases. In a group, the valence electrons keep the same effective nuclear charge, but now the orbitals are farther from the nucleus. Therefore, the nucleus has less of a pull on the outer electrons and the atomic radii are larger.
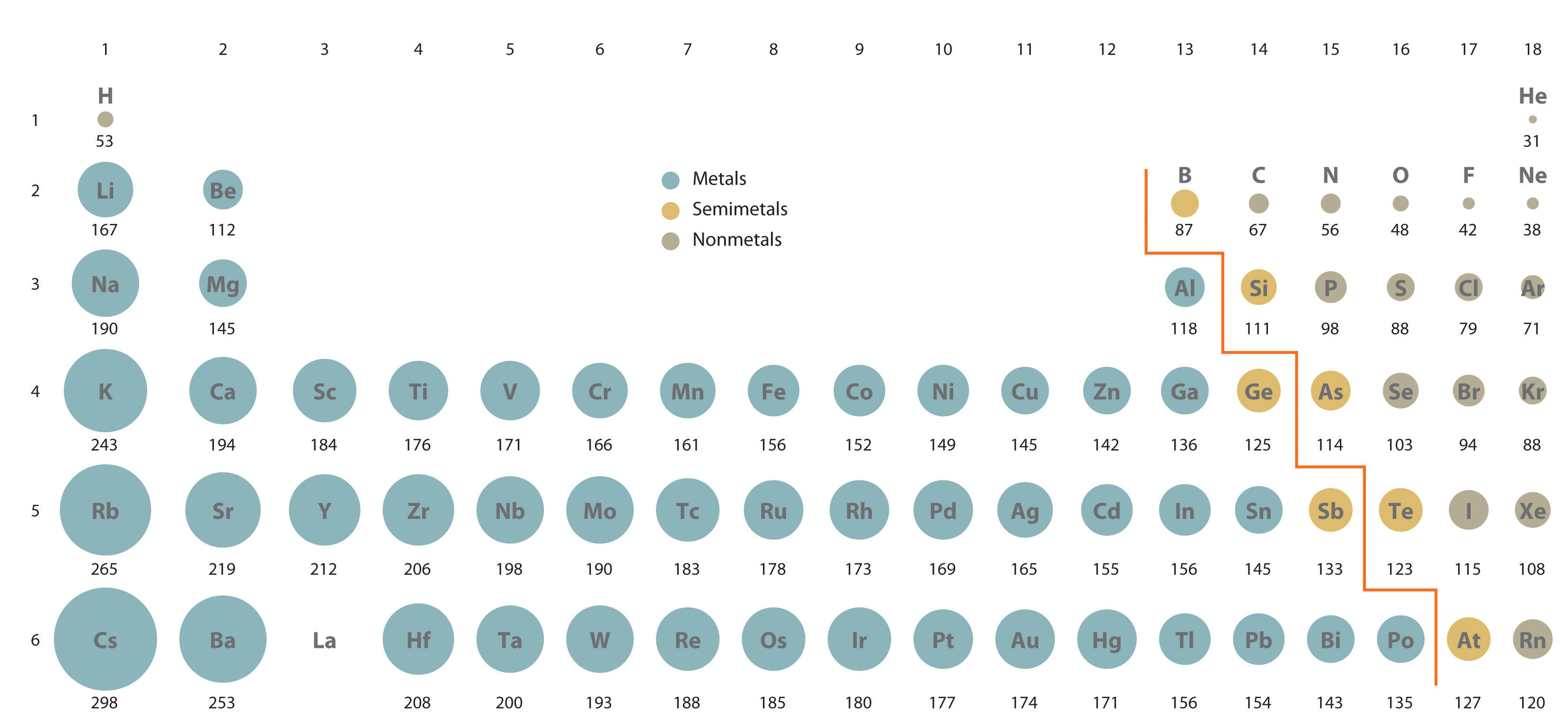
Figure \(\PageIndex{4}\) Calculated Atomic Radii (in Picometers) of the s-, p-, and d-Block Elements The sizes of the circles illustrate the relative sizes of the atoms. The calculated values are based on quantum mechanical wave functions.
Example \(\PageIndex{1}\)
Ranks the following in increasing atomic radius. Justify your answer by calculating effective nuclear charge and without looking at the figure above.
A) S, Mg, Al
B) Ba, Mg, Be
Strategy: Calculate the trend effective nuclear charge.
Solution:
A) S: 16-10 = +6
Mg: 12-10 = +2
Al: 13-10= +3
The greater the effective nuclear charge the more tightly an electron is held in by the nucleus because there is less electron shield between the valence electrons and the nucleus, and so, the smaller the radius.
So: S < Al < Mg
B) Ba: 56 - 54 = +2
Mg: 12 - 10 = +2
Be: 4 - 2 = +2
In the same group, the effective nuclear charge does not change. However, as you go down the periodic table, there are more filled shells, so the valence electron are further away from the nucleus, and so are not as tightly held in, increasing the atomic radius.
So: Be < Mg < Ba
Exercise \(\PageIndex{1}\)
Arrange these atoms in order of decreasing effective nuclear charge by the valence electrons: Si, Al, Mg, S
- Answer
-
Answer: S > Si > Al > Mg.
Explanation: The electrons above a closed shell are shielded by the closed shell. S has 6 electrons above a closed shell, so each one feels the pull of 6 protons in the nucleus.
Exercise \(\PageIndex{2}\)
An atom with an atomic radius smaller than that of sulfur (S) is __________.
A.) Oxygen (O)
B.) Chlorine (Cl)
C.) Calcium (Ca)
D.) Lithium (Li)
E.) None of the above
- Answer
-
C.) Oxygen (O)
Explanation: Periodic trends indicate that atomic radius increases up a group and from left to right across a period. Therefore, oxygen has a smaller atomic radius sulfur.
Exercise \(\PageIndex{3}\)
Identify the following statements as True/False.
A) Nitrogen has a larger atomic radius than oxygen.
B) A nonmetal has a smaller ionic radius compared with a metal of the same period.- Answer
-
A) True
Explanation: Atomic radius decreases from left to right on the periodic table. Therefore, nitrogen is larger than oxygen.
B) False
Explanation: The reasoning behind this lies in the fact that a metal usually loses an electron in becoming an ion while a non-metal gains an electron. This results in a smaller ionic radius for the metal ion and a larger ionic radius for the non-metal ion.
Atomic Radius of Ions
We can now use these concept to explain the atomic radius differences of cations and anions. A cation is an atom that has lost one of its outer electrons. Cations have a smaller radius than the atom that they were formed from. With the loss of an electron, the positive nuclear charge out powers the negative charge that the electrons exert. Therefore, the positive nucleus pulls the electrons tighter and the radius is smaller. An anion is an atom that has gained an outer electron. Anions have a greater radius than the atom that they were formed from. The gain of an electron does not alter the nuclear charge, but the addition of an electron causes a decrease in the effective nuclear charge. Therefore, the electrons are held more loosely and the atomic radius is increased.

Figure 5: Courtesy of Jessica Thornton (UCD)
A comparison of ionic radii with atomic radii (Figure \(\PageIndex{5}\)) shows that a cation, having lost an electron, is always smaller than its parent neutral atom, and an anion, having gained an electron, is always larger than the parent neutral atom.
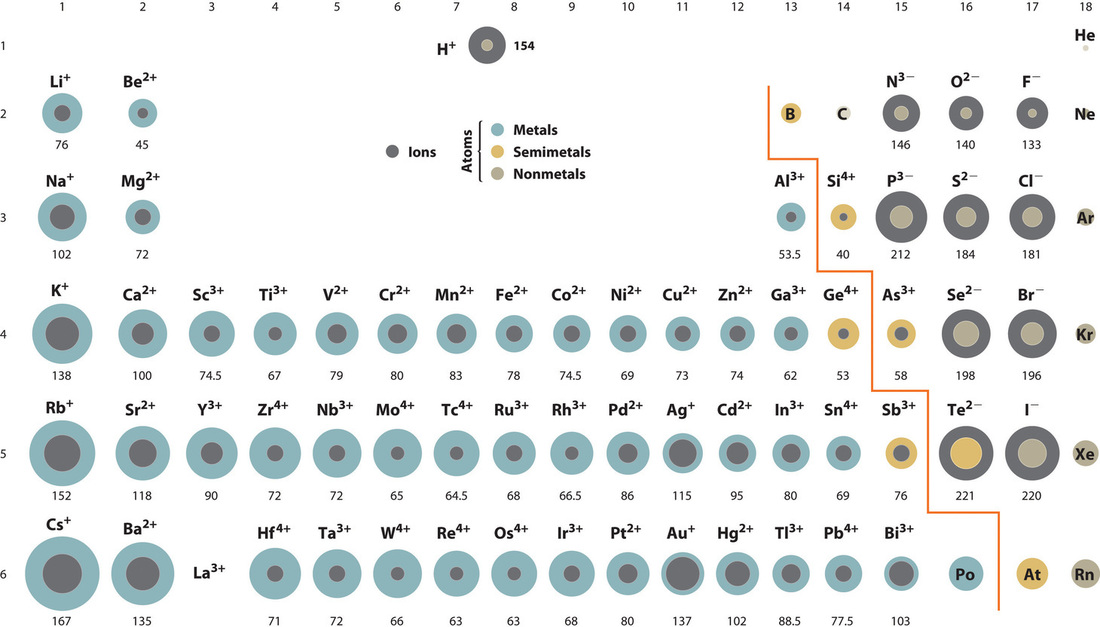
Figure \(\PageIndex{5}\): Ionic Radii (in Picometers) of the Most Common Ionic States of the s-, p-, and d-Block Elements.Gray circles indicate the sizes of the ions shown; colored circles indicate the sizes of the neutral atoms. Source: Ionic radius data from R. D. Shannon, “Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides,” Acta Crystallographica 32, no. 5 (1976): 751–767.
Ionic radii follow the same vertical trend as atomic radii; that is, for ions with the same charge, the ionic radius increases going down a column. The reason is the same as for atomic radii: shielding by filled inner shells produces little change in the effective nuclear charge felt by the outermost electrons. Again, principal shells with larger values of n lie at successively greater distances from the nucleus.
Because elements in different columns tend to form ions with different charges, it is not possible to compare ions of the same charge across a row of the periodic table. Instead, elements that are next to each other tend to form ions with the same number of electrons but with different overall charges because of their different atomic numbers. Such a set of species is known as an isoelectronic series. For example, the isoelectronic series of species with the neon closed-shell configuration (1s22s22p6).
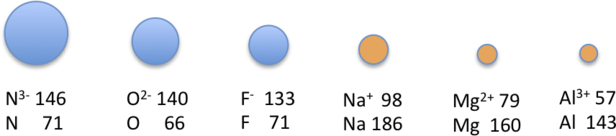
The sizes of the ions in this series decrease smoothly from N3− to Al3+. All six of the ions contain 10 electrons in the 1s, 2s, and 2p orbitals, but the nuclear charge varies from +7 (N) to +13 (Al). As the positive charge of the nucleus increases while the number of electrons remains the same, there is a greater electrostatic attraction between the electrons and the nucleus, which causes a decrease in radius. Consequently, the ion with the greatest nuclear charge (Al3+) is the smallest, and the ion with the smallest nuclear charge (N3−) is the largest. The neon atom in this isoelectronic series is not listed in Figure\(\PageIndex{5}\), because neon forms no covalent or ionic compounds and hence its radius is difficult to measure.
Ionization Energy (ionization potential)
Expelling an electron from an atom requires enough energy to overcome the magnetic pull of the positive charge of the nucleus. Therefore, ionization energy (I.E. or I) is the energy required to completely remove an electron from a gaseous atom or ion. The Ionization Energy is always positive.
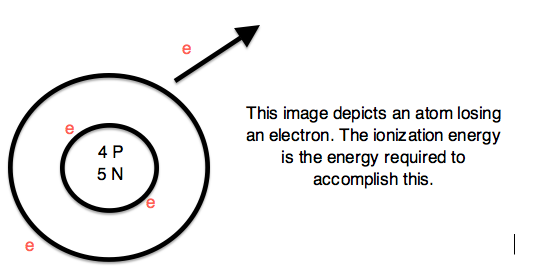
Figure 6: Courtesy of Jessica Thornton (UCD)
The energy required to remove one valence electron is the first ionization energy, the second ionization energy is the energy required to remove a second valence electron, and so on.
- 1st ionization energy
\[Na_{(g)} \rightarrow Na^+_{(g)}+ e^-_{(g)}\]
- 2nd ionization energy
\[Na^+_{(g)} \rightarrow Na^{2+}_{(g)} + e^-\]
Ionization energies increase relative to high effective charge. The highest ionization energies are the noble gases because they all have high effective charge due to their octet formation and require a high amount of energy to destroy that stable configuration. The highest amount of energy required occurs with the elements in the upper right hand corner. Additionally, elements in the left corner have a low ionization energy because losing an electron allows them to have the noble gas configuration. Therefore, it requires less energy to remove one of their valence electrons
| Element | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| Na | 496 | 4562 | |||||
| Mg | 738 | 1451 | 7733 | ||||
| Al | 577 | 1817 | 2745 | 11580 | |||
| Si | 786 | 1577 | 3232 | 4356 | 16090 | ||
| P | 1060 | 1903 | 2912 | 4957 | 6274 | 21270 | |
| S | 999.6 | 2251 | 3361 | 4564 | 7013 | 8496 | 27110 |
| Cl | 1256 | 2297 | 3822 | 5158 | 6542 | 9362 | 11020 |
| Ar | 1520 | 2666 | 3931 | 5771 | 7238 | 8781 | 12000 |
These are the ionization energies for the period three elements.
Notice how the second ionization energy for sodium went up dramatically. As did the third I.E. energy for magnesium, and the fourth I.E. for aluminum, and so on, all have a huge increase in energy compared to the proceeding one. This occurs because the proceeding configuration was in a stable octet formation; therefore it requires a much larger amount of energy to ionize.
For example, it is relatively easier to remove the first electrons of magnesium
Mg → Mg+ + e- I.E = 738 kJ/mol
It is a bit more challenging to remove the second electron requiring a little less than double the energy.
Mg+ → Mg+2 + e- I.E = 1451 kJ/mol
But become very difficult to remove the third electro requiring a little over five times the energy.
Mg+2 → Mg+3 + e- I.E = 7733 kJ/mol
That is because when magnesium loses two electrons, it is isoelectronic with the nearest noble gas, neon. To disturb the stability of atom with a stable noble gas configration requires much more energy.
In general, ionization energies increase going left to right across a period and increase going up a group. As you go up a group, the ionization energy increases, because there are less electron shielding the outer electrons from the pull of the nucleus. Therefore, it requires more energy to out power the nucleus and remove an electron. As we move across the periodic table from left to right, the ionization energy increases , due to the effective nuclear charge increasing. This is because the larger the effective nuclear charge, the stronger the nucleus is holding onto the electron and the more energy it takes to release an electron.

Figure 7: Courtesy of Jessica Thornton (UCD)
The ionization energy is only a general rule. There are some instances when this trend does not prove to be correct. These can typically be explained by their electron configuration. For example, Magnesium has a higher ionization energy than Aluminum. Magnesium has an electron configuration of [Ne]3s2. Magnesium has a high ionization energy because it has a filled 3s orbital and it requires a higher amount of energy to take an electron from the filled orbital.
Exercise \(\PageIndex{4}\)
Based on the periodic trends for ionization energy, which element has the highest ionization energy?
- Fluorine (F)
- Nitrogen (N)
- Helium (He)
- Answer
-
C.) Helium (He)
Explanation: Helium (He) has the highest ionization energy because, like other noble gases, helium's valence shell is full. Therefore, helium is stable and does not readily lose or gain electrons.
Electron Affinity
Electron affinity (E.A.) is the energy change that occurs when an electron is added to a gaseous atom. Electron affinity can further be defined as the enthalpy change that results from the addition of an electron to a gaseous atom. It can be either positive or negative value. The greater the negative value, the more stable the anion is.
- (Exothermic) The electron affinity is negative
\[X_{(g)} + e^- \rightarrow X^- + \text{Energy}\]
- (Endothermic) The electron affinity is positive
\[X_{(g)} + e^- + \text{Energy} \rightarrow X^- \]
First Electron Affinity
Ionization energies are always concerned with the formation of positive ions. Electron affinities are the negative ion equivalent, and their use is almost always confined to elements in groups 16 and 17 of the Periodic Table. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous -1 ions. It is the energy released (per mole of X) when this change happens. First electron affinities have negative values. For example, the first electron affinity of chlorine is -349 kJ mol-1. By convention, the negative sign shows a release of energy.
When an electron is added to a metal element, the process is not as exothermic (not as negative) and so less likely to occur. Metals have a less likely chance to gain electrons because it is easier to lose their valance electrons and form cations. It is easier to lose their valence electrons because metals' nuclei do not have a strong pull on their valence electrons. Thus, metals are known to have lower electron affinities.
Example \(\PageIndex{1}\): Group 1 Electron Affinities
This trend of lower electron affinities for metals is described by the Group 1 metals:
- Lithium (Li): -60 KJ mol-1
- Sodium (Na): -53 KJ mol-1
- Potassium (K): -48 KJ mol-1
- Rubidium (Rb): -47 KJ mol-1
- Cesium (Cs): -46 KJ mol-1
Notice that electron affinity decreases down the group because the electron affinity is becoming less negative (less exothermic) and less likely to gain an electron.
When nonmetals gain electrons, the energy change is usually much more negative because they give off energy to form an anion (exothermic process); thus, the electron affinity will be relatively more negative. Nonmetals have a greater electron affinity than metals because of their atomic structures: first, nonmetals have more valence electrons than metals do, thus it is easier for the nonmetals to gain electrons to fulfill a stable octet and secondly, the valence electron shell is closer to the nucleus, thus it is harder to remove an electron and it easier to attract electrons from other elements (especially metals). Thus, nonmetals have a higher electron affinity than metals, meaning they are more likely to gain electrons than atoms with a lower electron affinity.
Example \(\PageIndex{2}\): Group 17 Electron Affinities
For example, nonmetals like the elements in the halogens series in Group 17 have a higher electron affinity(more negative values) than the metals, meaning they are more likely to gain electrons than metals. This trend is described as below. Notice the negative sign for the electron affinity which shows that energy is released.
- Fluorine (F) -328 kJ mol-1
- Chlorine (Cl) -349 kJ mol-1
- Bromine (Br) -324 kJ mol-1
- Iodine (I) -295 kJ mol-1
Notice that electron affinity decreases (becomes less negative) down the group, meaning as you go down the group, the atom is less and less likely to gain an electron. We will discuss the Flourine/Chlorine anomaly below.
Nonmetals vs. Metals
To summarize the difference between the electron affinity of metals and nonmetals (Figure \(\PageIndex{1}\)):
- Metals: Metals like to lose valence electrons to form cations to have a fully stable octet. They absorb energy (endothermic) to lose electrons. The electron affinity of metals is lower than that of nonmetals.
- Nonmetals: Nonmetals like to gain electrons to form anions to have a fully stable octet. They release energy (exothermic) to gain electrons to form an anion; thus, electron affinity of nonmetals is higher than that of metals.
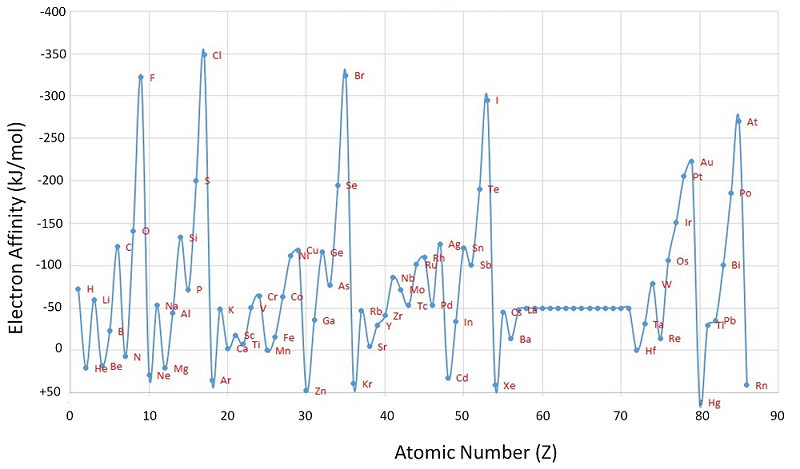
Figure \(\PageIndex{1}\): A Plot of Periodic Variation of Electron Affinity with Atomic Number for the First Six Rows of the Periodic Table. Notice that electron affinities can be both negative and positive. Image used with permission from Robert J. Lancashire (University of the West Indies).
Patterns in Electron Affinity
Going from left to right on the periodic table, the effective nuclear charge increases, making it more difficult to lose an electron, but making it easier to gain an electron. That is why we observe an increase in electron affinity as we move from left to right on the periodic table. When are looking a groups, we notic
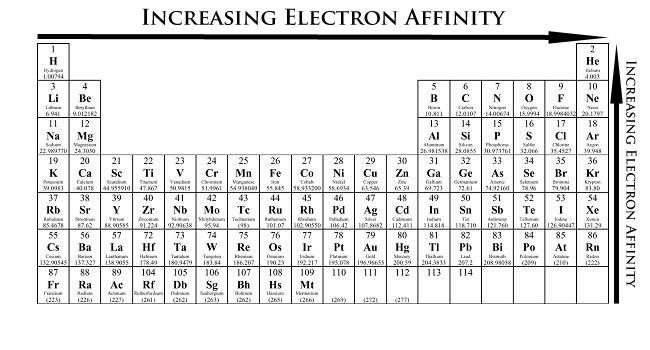
Periodic Table showing Electron Affinity Trend
Electron affinity increases upward for the groups and from left to right across periods of a periodic table because the electrons added to energy levels become closer to the nucleus, thus a stronger attraction between the nucleus and its electrons. Remember that greater the distance, the less of an attraction; thus, less energy is released when an electron is added to the outside orbital. In addition, the more valence electrons an element has, the more likely it is to gain electrons to form a stable octet. The less valence electrons an atom has, the least likely it will gain electrons.
Electron affinity decreases down the groups and from right to left across the periods on the periodic table because the electrons are placed in a higher energy level far from the nucleus, thus a decrease from its pull. However, one might think that since the number of valence electrons increase going down the group, the element should be more stable and have higher electron affinity. One fails to account for the shielding affect. As one goes down the period, the shielding effect increases, thus repulsion occurs between the electrons. This is why the attraction between the electron and the nucleus decreases as one goes down the group in the periodic table.
As you go down the group, first electron affinities become less (in the sense that less energy is evolved when the negative ions are formed). Fluorine breaks that pattern, and will have to be accounted for separately. The electron affinity is a measure of the attraction between the incoming electron and the nucleus - the stronger the attraction, the more energy is released. The factors which affect this attraction are exactly the same as those relating to ionization energies - nuclear charge, distance and screening. The increased nuclear charge as you go down the group is offset by extra screening electrons. Each outer electron in effect feels a pull of 7+ from the center of the atom, irrespective of which element you are talking about.
Example \(\PageIndex{3}\): Fluorine vs. Chlorine
A fluorine atom has an electronic structure of 1s22s22px22py22pz1. It has 9 protons in the nucleus.The incoming electron enters the 2-level, and is screened from the nucleus by the two 1s2 electrons. It therefore feels a net attraction from the nucleus of 7+ (9 protons less the 2 screening electrons).
In contrast, chlorine has the electronic structure 1s22s22p63s23px23py23pz1 with 17 protons in the nucleus. But again the incoming electron feels a net attraction from the nucleus of 7+ (17 protons less the 10 screening electrons in the first and second levels). There is also a small amount of screening by the 2s electrons in fluorine and by the 3s electrons in chlorine. This will be approximately the same in both these cases and so does not affect the argument in any way (apart from complicating it!).
The over-riding factor is therefore the increased distance that the incoming electron finds itself from the nucleus as you go down the group. The greater the distance, the less the attraction and so the less energy is released as electron affinity.
Comparing fluorine and chlorine is not ideal, because fluorine breaks the trend in the group. However, comparing chlorine and bromine, say, makes things seem more difficult because of the more complicated electronic structures involved. What we have said so far is perfectly true and applies to the fluorine-chlorine case as much as to anything else in the group, but there's another factor which operates as well which we haven't considered yet - and that over-rides the effect of distance in the case of fluorine.
Why is Fluorine an Anomaly?
The incoming electron is going to be closer to the nucleus in fluorine than in any other of these elements, so you would expect a high value of electron affinity. However, because fluorine is such a small atom, you are putting the new electron into a region of space already crowded with electrons and there is a significant amount of repulsion. This repulsion lessens the attraction the incoming electron feels and so lessens the electron affinity. A similar reversal of the expected trend happens between oxygen and sulfur in Group 16. The first electron affinity of oxygen (-142 kJ mol-1) is smaller than that of sulfur (-200 kJ mol-1) for exactly the same reason that fluorine's is smaller than chlorine's.
Comparing Group 16 and Group 17 values
As you might have noticed, the first electron affinity of oxygen (\(-142\; kJ\; mol^{-1}\)) is less than that of fluorine (\(-328\; kJ\; mol^{-1}\)). Similarly sulfur's (\(-200\; kJ\; mol^{-1}\)) is less than chlorine's (\(-349\; kJ\; mol^{-1}\)). Why? It's simply that the Group 16 element has 1 less proton in the nucleus than its next door neighbor in Group 17. The amount of screening is the same in both. That means that the net pull from the nucleus is less in Group 16 than in Group 17, and so the electron affinities are less.
The reactivity of the elements in group 17 falls as you go down the group - fluorine is the most reactive and iodine the least. Often in their reactions these elements form their negative ions. The first impression that is sometimes given that the fall in reactivity is because the incoming electron is held less strongly as you go down the group and so the negative ion is less likely to form. That explanation looks reasonable until you include fluorine!
An overall reaction will be made up of lots of different steps all involving energy changes, and you cannot safely try to explain a trend in terms of just one of those steps. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions more than makes up for the lower amount of energy released as electron affinity.
Second Electron Affinity
You are only ever likely to meet this with respect to the group 16 elements oxygen and sulfur which both form -2 ions. The second electron affinity is the energy required to add an electron to each ion in 1 mole of gaseous 1- ions to produce 1 mole of gaseous 2- ions. This is more easily seen in symbol terms.
\[ X^- (g) + e^- \rightarrow X^{-2} (g) \label{3}\]
It is the energy needed to carry out this change per mole of \(X^-\).
Why is energy needed to do this? You are forcing an electron into an already negative ion. It's not going to go in willingly!
\[ O_{g} + e^- \rightarrow O^- (g) \;\;\; \text{1st EA = -142 kJ mol}^{-1} \label{4}\]
\[ O^-_{g} + e^- \rightarrow O^{2-} (g) \;\;\; \text{2nd EA = +844 kJ mol}^{-1} \label{5}\]
The positive sign shows that you have to put in energy to perform this change. The second electron affinity of oxygen is particularly high because the electron is being forced into a small, very electron-dense space.
Exercise \(\PageIndex{5}\)
Rewrite the following list in order of decreasing electron affinity: fluorine (F), phosphorous (P), sulfur (S), boron (B).
- Answer
-
Fluorine (F)>Sulfur (S)>Phosphorous (P)>Boron (B)
Explanation: Electron affinity generally increases from left to right and from bottom to top.
Electronegativity Trends
Electronegativity can be understood as a chemical property describing an atom's ability to attract and bind with electrons. Because electronegativity is a qualitative property, there is no standardized method for calculating electronegativity. However, the most common scale for quantifying electronegativity is the Pauling scale (Table A2), named after the chemist Linus Pauling. The numbers assigned by the Pauling scale are dimensionless due to the qualitative nature of electronegativity. Electronegativity values for each element can be found on certain periodic tables. An example is provided below.
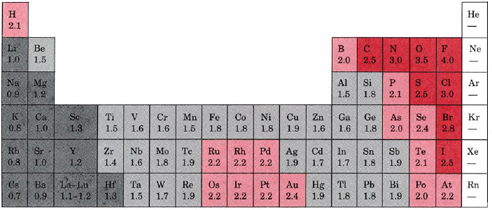
Figure 1: Periodic Table of Electronegativity values
Electronegativity measures an atom's tendency to attract and form bonds with electrons. This property exists due to the electronic configuration of atoms. Most atoms follow the octet rule (having the valence, or outer, shell comprise of 8 electrons). Because elements on the left side of the periodic table have less than a half-full valence shell, the energy required to gain electrons is significantly higher compared with the energy required to lose electrons. As a result, the elements on the left side of the periodic table generally lose electrons when forming bonds. Conversely, elements on the right side of the periodic table are more energy-efficient in gaining electrons to create a complete valence shell of 8 electrons. The nature of electronegativity is effectively described thus: the more inclined an atom is to gain electrons, the more likely that atom will pull electrons toward itself.
- From left to right across a period of elements, electronegativity increases. If the valence shell of an atom is less than half full, it requires less energy to lose an electron than to gain one. Conversely, if the valence shell is more than half full, it is easier to pull an electron into the valence shell than to donate one.
- From top to bottom down a group, electronegativity decreases. This is because atomic number increases down a group, and thus there is an increased distance between the valence electrons and nucleus, or a greater atomic radius.
- Important exceptions of the above rules include the noble gases, lanthanides, and actinides. The noble gases possess a complete valence shell and do not usually attract electrons. The lanthanides and actinides possess more complicated chemistry that does not generally follow any trends. Therefore, noble gases, lanthanides, and actinides do not have electronegativity values.
- As for the transition metals, although they have electronegativity values, there is little variance among them across the period and up and down a group. This is because their metallic properties affect their ability to attract electrons as easily as the other elements.
According to these two general trends, the most electronegative element is fluorine, with 3.98 Pauling units.
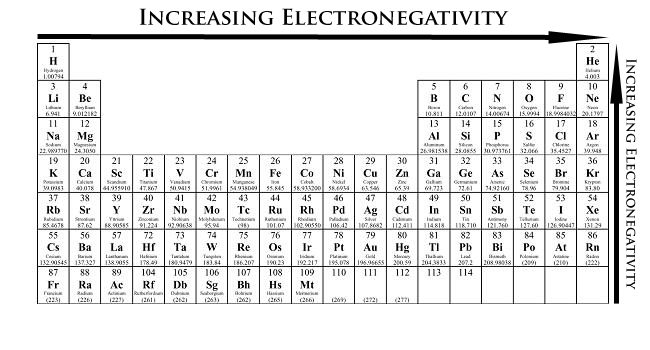
Figure 2. Periodic Table showing Electronegativity Trend
Exercise \(\PageIndex{6}\)
Which element is more electronegative, sulfur (S) or selenium (Se)?
Answer
-
Sulfur (S)
Explanation: Note that sulfur and selenium share the same column. Electronegativity increases up a column. This indicates that sulfur is more electronegative than selenium.
Exercise \(\PageIndex{7}\)
Why is the electronegativity value of most noble gases zero?
- Answer
-
Most noble gases have full valence shells.
Explanation: Because of their full valence electron shell, the noble gases are extremely stable and do not readily lose or gain electrons.
Exercise \(\PageIndex{8}\)
What is the difference between electronegativity and electron affinity?
- Answer
-
Although the trends are both related for similar reasons, electron affinity refers to the energy that is requires to completely gain an electron whereas electronegativity refers to the "pull" of the electrons towards an atom in bond.
Metallic Character
The metallic character is used to define the chemical properties that metallic elements present. Generally, metals tend to lose electrons to form cations. Nonmetals tend to gain electrons to form anions. They also have a high oxidation potential therefore they are easily oxidized and are strong reducing agents. Metals also form basic oxides; the more basic the oxide, the higher the metallic character.
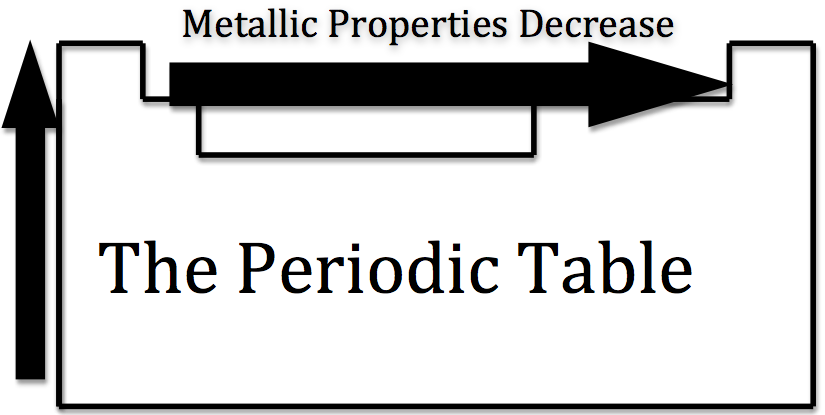
Figure 10: Courtesy of Jessica Thornton (UCD)
As you move across the table from left to right, the metallic character decreases, because the elements easily accept electrons to fill their valance shells. Therefore, these elements take on the nonmetallic character of forming anions. As you move up the table, the metallic character decreases, due to the greater pull that the nucleus has on the outer electrons. This greater pull makes it harder for the atoms to lose electrons and form cations.
Exercise \(\PageIndex{9}\)
Which has more metallic character, Lead (Pb) or Tin (Sn)?
- Answer
-
Lead (Pb)
Explanation: Lead and tin share the same column. Metallic character increases down a column. Lead is under tin, so lead has more metallic character.
Other Trends
Melting Points: Trends in melting points and molecular mass of binary carbon-halogen compounds and hydrogen halides are due to intermolecular forces. Melting destroys the arrangement of atoms in a solid, therefore the amount of heat necessary for melting to occur depends on the strength of attraction between the atoms. This strength of attraction increases as the number of electrons increase. Increase in electrons increases bonding.
Example: Melting point of HF should be approximately -145 °C based off melting points of HCl, HBr, and HI, but the observed value is -83.6°C.
Heat and electricity conductibility vary regularly across a period. Melting points may increase gradually or reach a peak within a group then reverse direction.
Example: Third period elements Na, Mg, and Al are good conductors of heat and electricity while Si is only a fair conductor and the nonmetals P, S, Cl and Ar are poor conductors.
Redox Potentials
Oxidation Potential
Oxidation is a reaction that results in the loss of an electron. Oxidation potential follows the same trends as the ionization energy. That is because the smaller the ionization energy, the easier it is to remove an electron. (e.g)
\[K_{(s)} \rightarrow K^+ + e^-\]
Reduction Potential
Reduction is a reaction that results in the gaining of an electron. Reduction potentials follow the same trend as the electron affinity. That is because the larger, negative electron affinity, the easier it is to give an electron. Example of Reduction:
\[F_{(s)} + e^- \rightarrow F^-\]
Contributors
- Original Source
- Modified by Ronia Kattoum (UA of Litte Rock)