6.2: Quantization: Planck, Einstein, Energy, and Photons
- Page ID
- 158441
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Explain how the study of Blackbody Radiation lead to the understanding of quantized energy
- Relate wavelength and frequency to the energy associated with electromagnetic radiation
- Summarize the photoelectric effect and its impact on discovering the photon
Introduction
In the last section we saw that c=\(\lambda \nu\), which means the frequency and wavelength of light are inversely related, that is \(\nu =\frac{c}{\lambda }\), and so doubling the wavelength halves the frequency. In this section, we shall define the concept of a photon of light and see how plank's equation relates the frequency and wavelength to the energy of light.
Blackbody Radiation*
One phenomenon that seemed to contradict the theories of classical physics was blackbody radiation, which is electromagnetic radiation given off by a hot object. The wavelength (i.e. color) of radiant energy emitted by a blackbody depends on only its temperature, not its surface or composition. Hence an electric stove burner or the filament of a space heater glows dull red or orange when heated, whereas the much hotter tungsten wire in an incandescent light bulb gives off a yellowish light.


Figure \(\PageIndex{1}\): Blackbody Radiation. When heated, all objects emit electromagnetic radiation whose wavelength (and color) depends on the temperature of the object. A relatively low-temperature object, such as a horseshoe forged by a blacksmith, appears red, whereas a higher-temperature object, such as the surface of the sun, appears yellow or white. Images used with permission from Wikipedia.
The intensity of radiation is a measure of the energy emitted per unit area. A plot of the intensity of blackbody radiation as a function of wavelength for an object at various temperatures is shown in Figure \(\PageIndex{2}\). One of the major assumptions of classical physics was that energy increased or decreased in a smooth, continuous manner. For example, classical physics predicted that as wavelength decreased, the intensity of the radiation an object emits should increase in a smooth curve without limit at all temperatures, as shown by the broken line for 6000 K in Figure \(\PageIndex{2}\). Thus classical physics could not explain the sharp decrease in the intensity of radiation emitted at shorter wavelengths (primarily in the ultraviolet region of the spectrum), which was referred to as the “ultraviolet catastrophe.” In 1900, however, the German physicist Max Planck (1858–1947) explained the ultraviolet catastrophe by proposing (in what he called "an act of despair") that the energy of electromagnetic waves is quantized rather than continuous. This means that for each temperature, there is a maximum intensity of radiation that is emitted in a blackbody object, corresponding to the peaks in Figure \(\PageIndex{2}\), so the intensity does not follow a smooth curve as the temperature increases, as predicted by classical physics. Thus energy could be gained or lost only in integral multiples of some smallest unit of energy, a quantum.
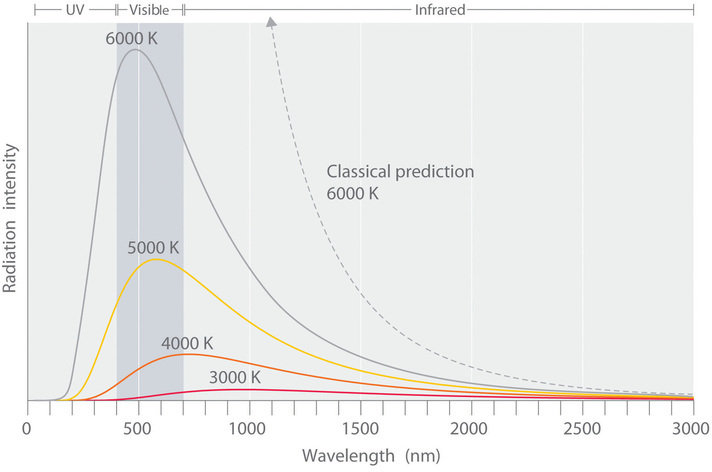
Figure \(\PageIndex{2}\): Relationship between the Temperature of an Object and the Spectrum of Blackbody Radiation it Emits. At relatively low temperatures, most radiation is emitted at wavelengths longer than 700 nm, which is in the infrared portion of the spectrum. The dull red glow of the electric stove element in Figure \(\PageIndex{1}\) is due to the small amount of radiation emitted at wavelengths less than 700 nm, which the eye can detect. As the temperature of the object increases, the maximum intensity shifts to shorter wavelengths, successively resulting in orange, yellow, and finally white light. At high temperatures, all wavelengths of visible light are emitted with approximately equal intensities. The white light spectrum shown for an object at 6000 K closely approximates the spectrum of light emitted by the sun (Figure \(\PageIndex{1}\)). Note the sharp decrease in the intensity of radiation emitted at wavelengths below 400 nm, which constituted the ultraviolet catastrophe. The classical prediction fails to fit the experimental curves entirely and does not have a maximum intensity.
Max Planck (1858–1947)
In addition to being a physicist, Planck was a gifted pianist, who at one time considered music as a career. During the 1930s, Planck felt it was his duty to remain in Germany, despite his open opposition to the policies of the Nazi government.

One of his sons was executed in 1944 for his part in an unsuccessful attempt to assassinate Hitler, and bombing during the last weeks of World War II destroyed Planck’s home. After WWII, the major German scientific research organization was renamed the Max Planck Society.
Although quantization may seem to be an unfamiliar concept, we encounter it frequently. For example, US money is integral multiples of pennies. Similarly, musical instruments like a piano or a trumpet can produce only certain musical notes, such as C or F sharp. Because these instruments cannot produce a continuous range of frequencies, their frequencies are quantized. Even electrical charge is quantized: an ion may have a charge of −1 or −2 but not −1.33 electron charges.
Planck accounted for this quantization by considering that the electromagnetic radiation that was emitted originated from vibrating atoms, called oscillators. He recognized that different particles could oscillate at different frequencies, and theses particles can either oscillate at that particular frequency or a whole-number multiple of it. As a result, the emitted radiation can only have certain energy, as given by Planck's Law.
\[E=nh\nu\]
Today Plank's constant is considered to be a fundamental physical constant and given a value of 6.626070040(81)×10−34J•s (joule-seconds), although in this class we will typically use it to 4 significant digits, ie., h = 6.626 × 10−34 J•s (joule-seconds)
If you consider n=1, which corresponds to then energy change from level to that of a lower level, then we can simplify Planck's Law:
\[E=h\nu\]
To understand blackbody radiation and figure 6.2.1 we need to rewrite Plank's law in terms of wavelength
\[E=h\nu =h\frac{c}{\lambda }\].
The blue shift in the wavelength is because as E goes up, \(\lambda\) goes down. The increase in energy is because as the temperature rises, n goes up.
Photoelectric Effect
The photoelectric effect was the observation that an electric current can be generated if light of certain frequencies hits the surface of a metal object, but that light of only certain colors can do this. In fact, for each type of metal there was a maximum wavelength that could eject an electron, and any light with a longer wavelength would have no effect, even if you increased its intensity. Einstein recognized that this could be explained by Plank's equation where one could define a "particle of light" or photon as having a fundamental energy of
\[E_{photon} = h\nu\]
in an electric field, it can kick an electron off the object and a current can flow. Einstein was able to use Plank's quantizations hypothesis to explain the photoelectric effect, where a "particle of light," a photon, had a characteristic energy described by Plank's constant
As can be showed in figure 6.2.2, a minimum energy of 2.0 eV is required to eject a photon off of potassium, and so red light would not work, while green and purple would.

Fig. 6.2.2: Potassium requires 2.0eV to eject an electron, and a photon of red light (700nm) only has 1.77eV and so no matter intense the red light is, it can not kick of an electron. Green and purple light have more energy, and when you shine them on the potassium you can kick off an electron.
The following video shows the photoelectric effect,
Video 6.2.1 (1:35 min YouTube), demonstrating the photoelectric effect. Credits go to College of Chemistry, University of California, Berkeley
Developed by Professor Alex Pines and Dr. Mark Kubinec with the support of The Camille & Henry Dreyfus Foundation
This second video by BestOfScience is a bit long, but summarizes this chapter
Video 6.2.2 (9:36 min YouTube) uploaded by BestOfScience. There are several sections of this video that may be useful. Around 20 seconds it starts going over spectrum. At around 2:25 it goes over black body radiation. At 5:40 it describes Plank's quantization hypothesis, and at 7:30 the photoelectric effect.
Example 6.2.1
What is the energy in joules and electron volts of a photon of 420-nm violet light?
Given: wavelength
Asked for: energy of single photon.
Strategy:
Use equation 6.2.3 to calculate the energy. Be mindful of the units being sure to convert the wavelength into meters.
Solution:
Photon energy is given by \[E = h\nu\] Since we are given the wavelength rather than the frequency, we solve the familiar relationship \(c = \nu\ \lambda\) for the frequency, yielding \[\nu\ = \dfrac{c}{\lambda}.\]
Now substituting known values yields
\[E = \dfrac{(6.63 \times 10^{-34} \, J \cdot s)(3.00 \times 10^8 \, m/s)}{420 \times 10^{-9} \, m} = 4.74 \times 10^{-19} \, J. \nonumber\]
Exercise 6.2.1
An x-ray generator, such as those used in hospitals, emits radiation with a wavelength of 1.544 Å.
- What is the energy in joules of a single photon?
- How many times more energetic is a single x-ray photon of this wavelength than a photon emitted by a ruby laser?
Answer:
- \(1.287 \times 10^{-15}\; J/photon\)
- 4497 times
Summary
- The fundamental building blocks of energy are quanta and of matter are atoms.
The properties of blackbody radiation, the radiation emitted by hot objects, could not be explained with classical physics. Max Planck postulated that energy was quantized and could be emitted or absorbed only in integral multiples of a small unit of energy, known as a quantum. The energy of a quantum is proportional to the frequency of the radiation; the proportionality constant h is a fundamental constant (Planck’s constant). Albert Einstein used Planck’s concept of the quantization of energy to explain the photoelectric effect, the ejection of electrons from certain metals when exposed to light. Einstein postulated the existence of what today we call photons, particles of light with a particular energy, E = hν. Both energy and matter have fundamental building blocks: quanta and atoms, respectively.
More Practice
CHEMTOUR : Click Chapter 7: Electromagnetic Radiation for a tutorial with interactive question set
Contributors
Modified by Joshua Halpern (Howard University)
- Modified by Bob Belford
- Blackbody Radiation Original Source*
- Ronia Kattoum

