2.7: Nomenclature of Ionic, Covalent, and Acid Compounds
- Page ID
- 158409
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)learning objectives
- Recognize ionic compound, molecular compounds, and acids.
- Give the names and formulas for ionic compounds
- Give the names and formulas for molecular compound s
- Give the name and formula for acidic compound
Introduction
Nomenclature, the naming of chemical compounds is of critical importance to the practice of chemistry, as a chemical can not only have many different names, but different chemicals can have the same name! So how can you do science if you do not know what you are talking about??? In this section we will look at nomenclature of simple chemical compounds. The rules we use depends on the type of compound we are attempting to name.
Overview of Nomenclature
The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. If it is a simple molecule we use Greek prefixes to identify the number of atoms of each type of element in the molecule. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. Note, hydrogen can not lose its only electron as then it would be a subatomic particle and the charge density would be too high, so it forms a covalent bond.
If the compound is ionic, we use the principle of charge neutrality to name the compound.
Figure\(\PageIndex{1}\):Outline of strategy for naming molecules (watch the Youtube immediately below for a lesson on how to use this outline)
Video\(\PageIndex{1}\): 2:17 min Youtube describing the logic of the flow diagram for nomenclature.
We will start with ionic compounds, then covalent and then acids.
Ionic Compounds
Ionic compounds have a [+] cation and a [-] anion. We use the Principle of Charge Neutrality, that is, for an ionic compound to be stable its chemical formula MUST BE NEUTRAL. So you do not need to state the number of cations and anions, you only need to state what they are (and what their charge is). You then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula.
We will start with binary ionic, which are between monatomic anions and cations. We note that there are two types of metals, those that have only 1 charge (Type 1), and those that can have more than one stable charge. Finally, polyatomic ions often form which are covalently bonded atoms where the total number of protons is not equal to the total number of electrons.
Binary Ionic
Binary ionic compounds are between a metal and nonmetal. This does not mean there are two atoms, but two types of atoms, so Al2S3 is a binary ionic compound. The rules are simple, name the cation first and the anion second, giving the anion the -ide suffix.
- Cation (metal) first
- Anion (nonmetal) second with -ide suffix
Example 1:
Na+ + Cl- = NaCl Ca2+ + 2Br- = CaBr2
Sodium + Chlorine = Sodium Chloride Calcium + Bromine = Calcium Bromide
Two Types of Monatomic Ions
As we have seen in the previous section, there are two types of monatomic ions, those of elements that form only one charge state, and those that can form multiple charged states. The anions are all of the first type, and gain electrons until they have the same number as the nearest noble gas. But metals form cations by losing electrons, and some metals form only one stable cation, while others can form many. For simplicity, we will call metals that form only one (invariant) charge state to be Type I and those that form variable charge states to be type 2. It is probably easiest to identify the Type 1 and consider others to be Type 2.
Type 1 Cations of Invariant Charge (Oxidation State)
There are some transition metals that form only one stable ion. Silver (Group 1B) forms a [+1] cation like the 1A alkali metals. Zinc and Cadmium (group 2B) form [+2] cations like the group 1B alkaline earth metals. The lighter Group 3A metals (Aluminum, Galium and Indium), along with Scandium and Yttrium lose 3 electrons to form [+3] cations. All the remaining transition metals form multiple charged ions (iron for example, forms Fe+2 and Fe+3 and thus has multiple charge states).
Figure\(\PageIndex{2}\) The elements in the above table form only one stable charged state. Note, we often refer to the charge of a monatomic ion as its oxidation state.
Video\(\PageIndex{2}\): 3:10 min YouTube on nomenclature for binary ionic compounds (with type I metal cations).
Using the Principle of Charge Neutrality (section 2.6.6) and knowing the charge of the ions allows you to determine the formula.
Exercise \(\PageIndex{1}\)
What is the formula for Barium Chloride?
- Answer
-
Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and always form the chloride ion of [-1]. For barium chloride to be neutral you would need two chlorides for every barium, and so the formula is BaCl2.
Exercise \(\PageIndex{2}\)
Name the following compound: Al2O3.
- Answer
-
Aluminum oxide. Al is a Type I monatomic ion, no Roman Numeral is needed since the charge does not change. Change the oxygen ending to the -ide ending for the anion.
Type II: Cations with Variable Charge (Oxidation State)
Most transition metals form multiple stable cations with different charges, and so you have to identify the charge state, which is done by writing the charge in Roman numerals and placing it in parenthesis after the name of the metal. So Fe+2 is iron(II) and Fe+3 is iron(III). Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the following table. Note, "-ic" stands for the higher oxidation state (charge), and "-ous" stands for the lower oxidation state.
Figure\(\PageIndex{3}\): Example of some compounds that have multiple oxidation states.
Note mercury(1) is not a monoatomic cation, but is really a
Example 2
| Ions: | Fe2++ 2Cl- | Fe3++ 3Cl- |
| Compound: | FeCl2 | FeCl3 |
| Nomenclature | Iron (II) Chloride | Iron (III) Chloride |
- -ous ending is used for the lower oxidation state
- -ic ending is used for the higher oxidation state
Example 3
| Compound | Cu2O | CuO | FeCl2 | FeCl3 |
| Charge | Charge of copper is +1 | Charge of copper is +2 | Charge of iron is +2 | Charge of iron is +3 |
| Nomenclature | Cuprous Oxide | Cupric Oxide | FerrousChloride | FerricChloride |
Some of the more common chemicals use the -ous/-ic nomenclature, but the use of Roman Numerals to designate the charge is acceptable.
Exercise \(\PageIndex{3}\)
Name the following compound: CoBr3.
- Answer
-
Cobalt(III) bromide. We know that Br has a -1 charge and the are three bromide ions. There is one cobalt ion. To counterbalance the -3 charge, the charge of the Co ions must be +3. So 1(x) + 3(-1) = 0. The "x" must be +3.
Polyatomic Ions
Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. They end in [-ide], [-ate] and [-ite]. Many of these are oxyanions with oxygen being bonded to a nonmetal and others are carboxylate ions. It should be noted that ionic comounds like ammonium acetate are composed entirely of nonmetals, but they form a crystal structure of cations and anions.
Figure \(\PageIndex{4}\): List of Polyatomic ions freshmen chemistry students are required to know.
Naming Oxyanions: Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1, nonmetals of the same periodic group from homologous oxyanions. Lets look at the 4 oxyanions of bromine
- perbromate (BrO4-)
- bromate (BrO3-)
- bromite (BrO2-)
- hypobromite (BrO-)
These all end with -ate or -ite, and the real reasoning deals with the oxidation state of the nonmetal, sort of its hypothetical charge within the covalently bonded polyatomic ion. We will cover that in a later chapter, but right now we note that the "-ate" ions have more oxygens and the "-ites" have less, with "per____ate" having the most, and "hypo___ite" having the least.
of the same family of the periodic table
Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6), Selenium and Tellurium form similar anions to Sulfur, and Arsenic forms similar ones to Phosphorous. See video 2.7.3 below.
Note: The second period of the periodic table have nonmetals that behave different than the rest. Nitrogen forms different oxyanions than phosphorous or Arsenic, Oxygen does not form oxyanions, and although I have seen perfluorate, fluorate and fluorite salts on an exam and webpages where they form similar structures to chlorine, I believe the only one that really exists is hypofluorite (FlO-).
So why is the second row an exception? We will understand later this semester that as you go down a family or group of the periodic table, the volume of the atoms increases. The reason the second row nonmetals are an exception can best be understood by their small size (how are you going to get 4 oxygens around a small fluorine)? But a search of the web will show you perfluorate, fluorate and fluorite, as if they form the same types of oxyanions as chlorine does, yet there is no perfluorite in a resource like pubchem, which as of 2018 has over 95 million chemical compounds. So on an exam at the freshmen level, I would probably treat them as if they follow the same trend as chlorine, because that is the schema you are learning.
Carboxylate ions: Another class of polyatomic anions are based on the carboxylate functional group of organic chemistry. The compounds phthlate, acetate and oxalate have this functional group. See video 2.7.4 below
Figure\(\PageIndex{4}\): Carboxylate functional group that is the bases for many organic ions.
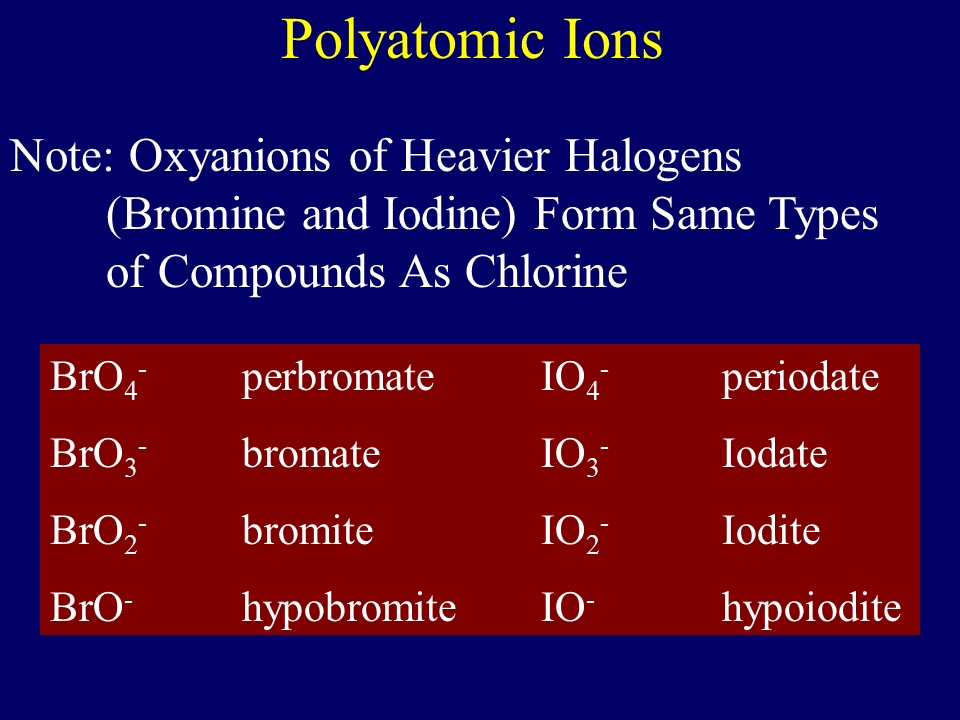
Figure\(\PageIndex{6}\): Slide showing periodic trends for nomenclature of oxyanions of halogens.
Figure 2.7.4 Shows several types of polyatomic anions, name
Understanding Oxyanions
This video can help you understand oxyanions
Video\(\PageIndex{3}\): 2:20 minute Youtube to help you remember names of oxyanions
Polyatomic Ions based on carboxylate functional group
Organic compounds have functional groups, and many organic anions are based on the carboxylate group. This video helps you use that to remember the formula of polyatomic ions like acetate, phthlate and oxylate.
Video\(\PageIndex{4}\): YouTube to help you remember names of
Exercise \(\PageIndex{4}\)
Name the following compounds:
A) NiCO3
B) Sr(NO2)2
C) NH4NO3
- Answer
-
A) Nickel(II) carbonate. Nickel is a Type II cation, so you need a Roman Numeral. To figure out that charge "x", we need take a look at what we know. There is one carbonate ion at a charge of -2. There is one Nickel ion at unknown charge "x". 1(x) + 1(-2) = 0. To be charge neutral, x=+2.
B) Strontium nitrite. Strontium is a type I cation and there is no need for a Roman Numeral. NO2- is the nitrite ion.
C) Ammonium nitrate. both are polyatomic ions, so you simply state the name of cation followed by the name of the anion.
Molecular Compound
| # of Atoms | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Prefixes | Mono- | Di- | Tri- | Tetra- | Penta- | Hexa- | Hepta- | Octa- | Nona- | Deca- |
Example 4
CO = carbon monoxide BCl3 = borontrichloride
CO2 = carbon dioxide N2O5 =dinitrogen pentoxide
The prefix mono- is not used for the first element. If there is not a prefix before the first element, it is assumed that there is only one atom of that element.
Exercise \(\PageIndex{5}\)
Laughing gas is commonly named as nitrous oxide, N2O. What is the systematic name for this compound?
- Answer
-
Dinitrogen monoxide. First, we must establish this a molecular compound before we use prefixes. Remember, prefixes are NOT used for ionic compounds.
Exercise \(\PageIndex{6}\)
Name the following compound: NO3
- Answer
-
Nitrogen trioxide. BE AWARE: This is not the the nitrate ion. If it were, it would a negative charge. It a molecular compound and named as such.
Binary Acids
Although HF can be named hydrogen fluoride, it is given a different name for emphasis that it is an acid.
Some common binary acids include:
It is very important to include (aq) after the acids because the same compounds can be written in gas phase with hydrogen named first followed by the anion ending with –ide.
Example 5
hypo____ite ____ite ____ate per____ate
ClO- ClO2- ClO3- ClO4-
hypochlorite chlorite chlorate perchlorate
---------------->
As indicated by the arrow, moving to the right, the following trends occur:
Increasing number of oxygen atoms
Increasing oxidation state of the nonmetal
(Usage of this example can be seen from the set of compounds containing Cl and O)
This occurs because the number of oxygen atoms are increasing from hypochlorite to perchlorate, yet the overall charge of the polyatomic ion is still -1. To correctly specify how many oxygen atoms are in the ion, prefixes and suffixes are again used.
Table: Common Polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. Such acids include
- If the ion ends in -ate and is added with an acid,
the acid name will have an -ic ending - If the ion ends in -ite and
- is added with an acid, then the acid name will have an -ous ending. Example: nitite ion (NO2-) + H+ (denoting formation of acid) = nitrous acid (HNO2)
Naming Acid Salts
Video 2.7.e: (1'28") Acid Salts 1: Youtube for naming compounds that can form from carboxylate (acid, salt and acid salt)
References
- Pettrucci, Ralph H. General Chemistry: Principles and Modern Applications. 9th. Upper Saddle River: Pearson Prentice Hall, 2007
- Nomenclature of Inorganic Chemistry, Recommendations 1990, Oxford:Blackwell Scientific Publications. (1990)
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. Electronic version..
- Biochemical Nomenclature and Related Documents, London:Portland Press, 1992.
Problems
1. What is the correct formula for Calcium Carbonate?
a. Ca+ + CO2-
b. CaCO2-
c. CaCO3
d. 2CaCO3
2. What is the correct name for FeO?
a. Iron oxide
b. Iron dioxide
c. Iron(III) oxide
d. Iron(II) oxide
3. What is the correct name for Al(NO3)3?
a. Aluminum nitrate
b. Aluminum(III) nitrate
c. Aluminum nitrite
d. Aluminum nitrogen trioxide
4. What is the correct formula of phosphorus trichloride?
a. P2Cl2
b. PCl3
c. PCl4
d. P4Cl2
5. What is the correct formula of lithium perchlorate?
a. Li2ClO4
b. LiClO2
c. LiClO
d. None of these
6. Write the correct name for these compounds.
a. BeC2O4:
b. NH4MnO4:
c. CoS2O3:
7. What is W(HSO4)5?
8. How do you write diphosphorus trioxide?
9. What is H3P?
10. By adding oxygens to the molecule in number 9, we now have H3PO4? What is the name of this molecule?
Answer
1.C; Calcium + Carbonate --> Ca2+ + CO32- --> CaCO3
2.D; FeO --> Fe + O2- --> Iron must have a charge of +2 to make a neutral compound --> Fe2+ + O2- --> Iron(II) Oxide
3.A; Al(NO3)3 --> Al3+ + (NO3-)3 --> Aluminum nitrate
4.B; Phosphorus trichloride --> P + 3Cl --> PCl3
5.D, LiClO4; Lithium perchlorate --> Li+ + ClO4- --> LiClO4
6. a. Beryllium Oxalate; BeC2O4 --> Be2+ + C2O42- --> Beryllium Oxalate
b. Ammonium Permanganate; NH4MnO4 --> NH4+ + MnO4- --> Ammonium Permanganate
c. Cobalt (II) Thiosulfate; CoS2O3 --> Co + S2O32- --> Cobalt must have +2 charge to make a neutral compund --> Co2+ + S2O32- --> Cobalt(II) Thiosulfate
7. Tungsten (V) hydrogen sulfate
8. P2O3
9. Hydrophosphoric Acid
10. Phosphoric Acid
----
Practice sheets
1402 Nomenclature Problem set 1 (Ionic and Covalent Compounds)
1402 Nomenclature Problem set 2 (Acids)
1)aluminum nitride AlN
4)nitrogen dioxide NO2
5)zinc sulfate ZnSO4
6)zinc sulfide ZnS
7)zinc phosphate Zn3(PO4)2
8)carbon tetrachloride CCl4
9)sodium hydroxide NaOH
10)barium hydroxide Ba(OH)2
11)titanium (II) cyanide Ti(CN)2
12)cadmium iodide CdI2
13)cadmium acetate Cd(CH3CO2)2
14)cadmium bromide CdBr2
15)cadmium nitrate Cd(NO3)2
16)cadmium sulfide CdS
17)phosphorus pentachloride PCl5
18)iron (II) sulfide FeS
19)iron (II) sulfate FeSO4
20)iron (III) sulfate Fe2(SO4)3
21) iron (III) nitrate Fe(NO3)3
22)magnesium nitride Mg3N2
23)magnesium nitrate Mg(NO3)2
25)tin (IV) fluoride SnF4
26)tin (II) fluoride SnF2
27)tin (IV) sulfide SnS2
28)tin (II) sulfide SnS
29)nitrogen trichloride NCl3
30)manganese (II) sulfide MnS
31)manganese (II) sulfate MnSO4
32)manganese (II) phosphate Mn3(PO4)2
- manganese (II) nitrate Mn(NO3)2
34)vanadium (V) oxide V2O5
35)molybdenum (VI) sulfide MoS3
36)silver phosphide Ag3P
37)mercury (II) oxide HgO
38)chromium (III) bromide CrBr3
39)chromium (III) nitrate Cr(NO3)3
40)sulfur dichloride SCl2
41)magnesium sulfide MgS
42)magnesium sulfate MgSO4
43)cobalt (III) carbonate Co2(CO3)3
44)cobalt (III) phosphide CoP
Write formulas from the following names:
1)Ni(NO3)3 nickel (III) nitrate
2)NiCl3 nickel (III) chloride
3)Mn(OH)2 manganese (II) hydroxide
4)Hg(CN)2 mercury (II) cyanide
5)CuS copper (II) sulfide
6)PCl3 phosphorus trichloride
7)Sr(NO3)2 strontium nitrate
8)FeSO4 iron (II) sulfate
9)Bi(OH)3 bismuth (III) hydroxide
10)AgHCO3 silver bicarbonate
11)Co2(CO3)3 cobalt (III) carbonate
12)SF2 sulfur difluoride
14)NCl ammonium chloride
15)(NH4)2SO4 ammonium sulfate
16)NiPO4 nickel (III) phosphate
17)Co(CN)2 cobalt (II) cyanide
18)NCl3 nitrogen trichloride
19)Hg2S mercury (I) sulfide
20)Hg3(PO4)2 mercury (II) phosphate
21) Mn(NO3)2 manganese (II) nitrate
22)Fe2(SO4)3 iron (III) sulfate
23)Sn(CN)2 tin (II) cyanide
24)ZnSO4 zinc sulfate
25) LiNO3 lithium nitrate
26) LiCN lithium cyanide
27) Ba(CN)2 barium cyanide
28) Al(CN)3 aluminum cyanide
29) CuCN copper (I) cyanide
30) Cu(CN)2 copper (II) cyanide
31) CuSO4 copper (II) sulfate
32) Cu(NO2)2 copper (II) nitrite
33) Cu(NO3)2 copper (II) nitrate
34) CuN copper (III) nitride
35) CuBr copper (I) bromide
36) CuBr2 copper (II) bromide
37) Cu2SO4 copper(I) sulfate
38) Cu2(SO4)3 copper (III) sulfate
39) Cu3PO4copper (I) phosphate
40) Cu3(PO4)2 copper (II) phosphate
41) PI5 phosphorous pentaiodide
42) PbS lead (II) sulfide
43) PbS2 lead (IV) sulfide
44) SnCl4 tin (IV) chloride
45) SnBr2 tin (II) bromide
46) SnSO4 tin (II) sulfate
47) Sn3(PO4)2 tin (II) phophate
48) SF6 sulfur hexafluoride
49) P4H10 tetraphosphorus decahydride
50) CuS copper (II) sulfide
51)HNO3 nitric acid
52)H2S hydrosulfuric acid
53)H3PO4 phosphoric acid
55)H2SO4 Þsulfuric acid
57)H2SO3 sulfurous acid
58)HClO4 perchloric acid
59)HNO2 nitrous acid
60) H3PO3 phosphorus acid
61) H3AsO4 arsenic acid
62) HIO2 iodous acid
63) H2C2O 4 oxalic acid
64) Arsenic acid H3AsO4
65) Acetic acid C2H4O2
66) hydrocyanic acid HCN
67) periodic acid HIO4
68) bromous acid HBrO2
69) hypoiodous iodic acid HIO
70) hypochlorous acid HClO
71) carbonic acid H2CO3
72) phosphoric acid H3PO4
73) arsenous acid H3AsO3
74) phosporous acid H3PO3
75)sulfurous acid H2SO3
76) hydrosulfuric acid H2S
1402 Nomenclature Problem set 1 Key
1402 Nomenclature Problem set 2 Key
Key to Practice Set 2
- HNO3 Þ nitric acid
- H2S Þ hydrosulfuric acid
- H3PO4 Þ phosphoric acid
- HBrO3 Þ bromic acid
- H2SO4 Þsulfuric acid
- HIO Þ hypoiodic acid
- H2SO3 Þ sulfurous acid
- HClO4 Þ perchloric acid
- HNO2 Þ nitrous acid
- H3PO3 Þ phosphorus acid
- H3AsO4 Þ arsenic acid
- HIO2 Þ iodous acid
- H2C2O 4 Þ oxalic acid
- Arsenic acid Þ H3AsO4
Acetic acid Þ C 2 H 4 O 2 - hydrocyanic acid Þ HCN
- periodic acid Þ HIO3
- bromous acid Þ HBrO2
- hypoiodous iodic acid Þ HIO
- hypochlorous acid Þ HClO
- carbonic acid Þ H2CO3
- phosphoric acid Þ H3PO4
arsenous acid Þ H 3 AsO 3 - phosporous acid Þ H3PO4
- sulfurous acid Þ H2SO3
- hydrosulfuric acid Þ H2S
Contributors
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
Modified by Ronia Kattoum (UA of Little Rock)

