2.6: Writing Formulas for Ionic Compounds
- Page ID
- 158408
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning objectives
- Relate the stability of ionic compounds to Coulomb’s Law
- Recognize properties of ionic compounds
- Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions
- Predict the charge of monatomic main group elements based on their group number.
- Name monoatomic anions and cations
- Memorize polyatomic ions
- Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality.
Introduction
The substances described in the preceding discussion are composed of molecules that are electrically neutral; that is, the number of positively-charged protons in the nucleus is equal to the number of negatively-charged electrons. In contrast, ions are atoms or assemblies of atoms that have a net electrical charge results from the gain or loss of electrons. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Conversely, ions that contain more electrons than protons have a net negative charge and are called anions. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. Neutral atoms can become ions by gaining or losing electrons.
Coulomb's Law
Coulomb's Law is a mathematical formula that describes the electrostatic interaction between two charges particles that was first proposed by the French physicist Charles Coulomb (1736–1806). Coulomb's law is often called an inverse square law, as the force decreases as a function of the square of the distance between the two particles.
\[ E= k\dfrac{q_1q_2}{ r} \; \; \; \; \; \; \; \; F= k\dfrac{q_1q_2}{r^2} \label{eq.11.0.1}\]
- \(E\) is the potential energy
- \(F\) is the force
- \(r\) is the distance of separation (note that the potential goes to zero when they are separated by infinity)
- \(q\) is the charge of the ions
- \(k\) is a constant
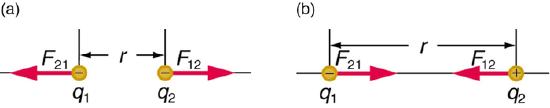
In (a) the like charges repel each other, while in (b) the opposite charges attract each other.
The electrostatic interactions can be understood by the above image, where in (a), like charges repel and in (b), opposite charges attract. If you look at Coulomb's law, you note that in (a) the force is positive (because both q1 and q2 are negative), while in (b) the force is negative (because q1 is negative while q2 is positive). That is, attractive interactions result in a lowering of the energy
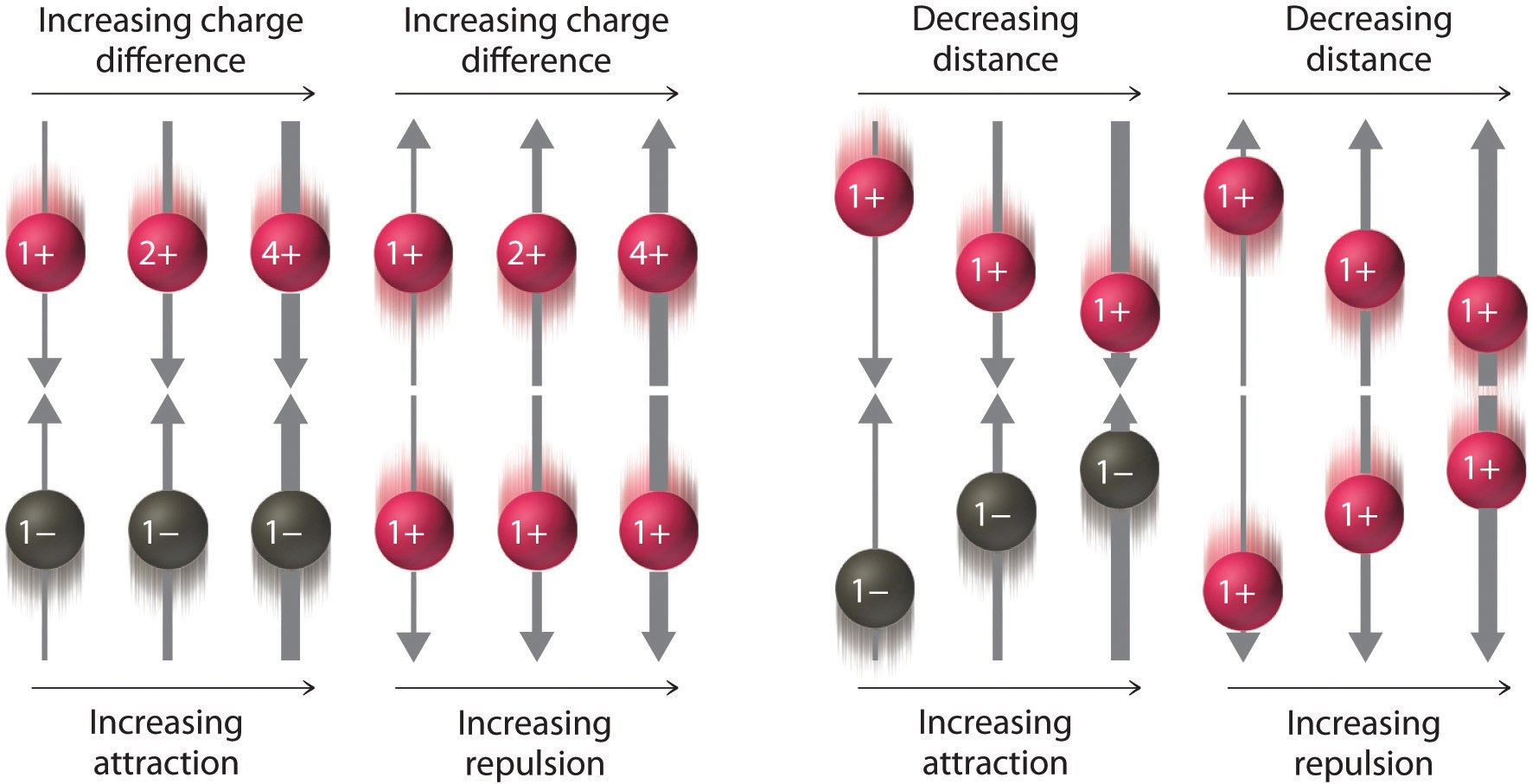
In the above diagram the width of the arrows indicate the magnitude of the forces of electrostatic interaction. You should be able to look at the algebra associated with Coulomb's Law and see how it results in these interactions. In the numerator is the charge, and so the greater the charge, the greater the interaction. In the denominator is the distance between the charges, and so the larger the distance, the weaker the interaction. It is very important that you learn how to "read" algebraic equations like Coulomb's Law.
Crystal Lattice and Principle of Charge Neutrality
Why must ionic compounds be neutral?
An ionic bond is not between two ions, but the result of the interaction of multiple cations (+ions) and anions (-ions). That is, a salt crystal has +/+ and -/- repulsions (increasing the energy), and +/- attractions (decreasing the energy), and if the attractive forces are greater than the repulsive, an ionic compound is formed. In the following diagram we see how a crystal forms which maximizes the attractive interactions of the opposite charges while minimizing the repulsive interactions of the like charges. If the compound was not neutral, but had more cations, or more anions, the like/like repulsive forces would be larger and the material would become unstable and break apart. Therefore, ionic compounds must be neutral.
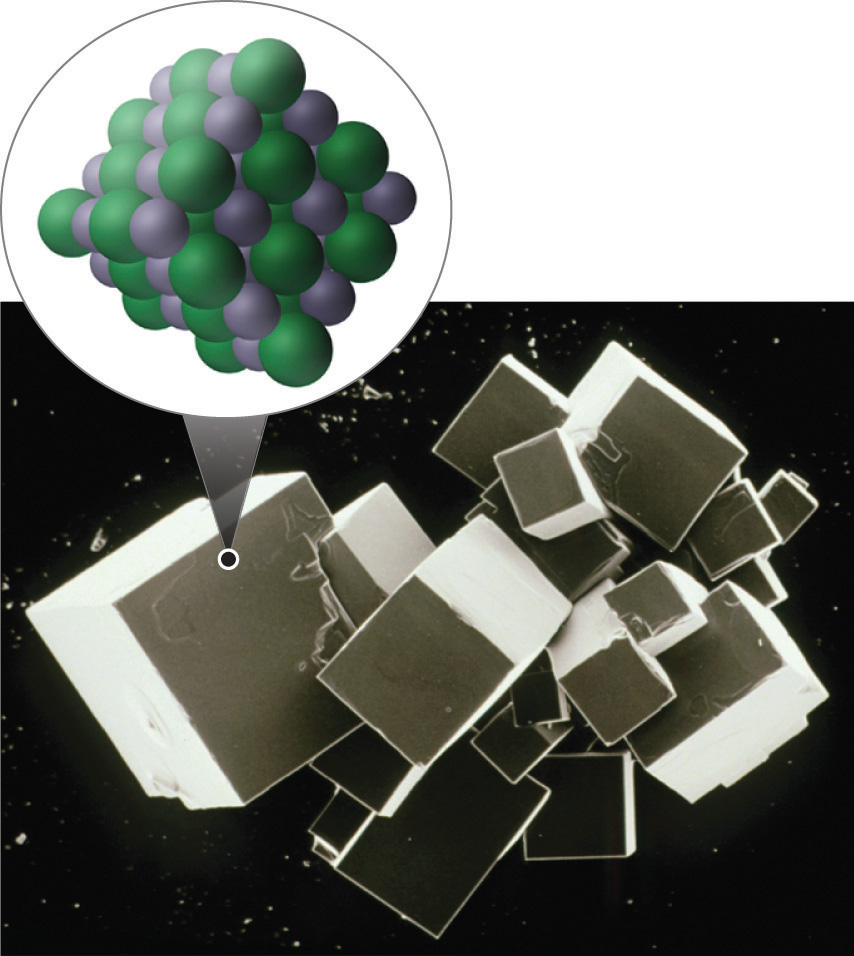
In the above image the sodium (a metal) loses and electron (becoming smaller) while the Chlorine atom (nonmetal) gains an electron (becoming bigger). A crystal lattice results where the cations and anions pack in three dimensional space that can result in a macroscopic crystal that is visible to the naked eye..
The salt crystals seen to the naked eye are the result of the way cations and anions pack to form a stable crystal structure that minimizes like/like repulsions and maximizes opposite charge attractions as defined by Coulomb's Law. The following video from YouTube describes the formation of sodium chloride.
YouTube video describing formation of sodium chloride, table salt.
Properties of Ionic Compounds
Because of the arrangement of cations and anions in a the crystal lattice, these ions are held tightly into their position. Although the ions can vibrate and oscillate about their position, it take much more energy than room temperature for one ion to break free of the lattice structure. This results in very high melting points and boiling points for ionic compounds. Therefore, ionic compounds are crystal solids at room temperature. They are not malleable or soft. When struck sharply, they do shatter cleanly, with layers of ions breaking off at once.
Monatomic Ions
Monatomic Cations: When atoms (typically metals) lose one or more electrons to become positively charged cations. For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, Na+ as shown in the following shorthand notation:
\[\ce{Na -> Na^{+} + e^{-}}\]
Another example shows the formation of the magnesium ion from the neutral magnesium atom losing 2 electrons to pick up a +2 charge.
\[\ce{Mg -> Mg^{2+} + 2e^{-}}\]
So, why does sodium lose one electron while magnesium loses two electrons? The charge of main group elements can be determined by the group number with the "A" designation. Metals in groups 1A-3A lose the same amount of electrons as in their group number. This results in atoms with the same number of electrons as the noble gas directly preceding the element (more details on this when we do electron configurations later in this text). When we name monatomic cations ions that form only on charge like those in Groups 1A-3A, we simply identify the name of the element followed by the word ion.
Ex: Na+ is named as the sodium ion to differentiate it from the neutral sodium atom, Na.
Note: Why does hydrogen not form a +1 cation?
Although hydrogen has one electron in its outer shell (like all the alkali metals), it only has one electron and so completely removing it would leave behind a bare nucleus which would not be stable. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. In the above video sodium with 11 electrons gave an electron to chlorine, forming the \(\ce{Na^{+}}\) cation and the \(\ce{Cl^{-}}\) anion, which then formed the crystal table salt NaCl. If chlorine was to try and remove the electron from hydrogen, it would not be able to as the resulting nucleus would pull it back, this results in the acid HCl, where the chlorine can not completely remove the electron from the hydrogen.
Monatomic Anions: when atoms (typically non-metals) gain one or electrons to become negatively charged anions. For example, when a neutral fluorine atom gains one electrons, it becomes the fluoride ion as shown by the following shorthand notation:
\[\ce{F + e^{-} → F^{-}}\]
Another example is the formation of the sulfide ion when a neutral sulfur atom gains two electrons picking up a -2 charge.
\[\ce{S + 2e^{-} → S^{2-}}\]
As you may have inferred, the position of the main group non-metal on the periodic table can also be used to predict its charge. If you subtract eight from the group number of the element in groups 5A-7A, you will be able to predict the charge of the anion.
Example: Sulfur is in group 6A. So, 6-8 = -2
This allows the atoms of a given nonmetal to gain enough electrons so as to have the same number of electrons as the noble gas following the element. When sulfur gains two electrons, it will have 18 electrons, like argon. We will go into the reasoning behind this in later chapters. Finally, when monatomic anions are named, you must change the -ine end of the element's name to the -ide ending following by the word ion.
For example, F- is named as fluoride ion to differentiate it from F, the fluorine atom.
To help you remember these patterns, think about metals (everything to the left of the stairs) as positively charged, and think about the non-metals (everything to the right of the stairs) as negatively charged. You may have noticed that the transition metals have more than one charge. The charge of the transition metals is not as easily determined as the main group elements. Because they can potentially have more than one charge, it is important to differentiate between ions of the same element by adding a Roman Numeral in parenthesis after the element name following by the word ion.
For example, iron can form two ions, \(\ce{Fe^{2+}}\) (named as iron(II) ion), and \(\ce{Fe^{3+}}\) (named as iron(III) ion).
Note, the older system assigned the ion with the lower charge with the -ous ending and the ion with the greater charge with the -ic ending.
Example: \(\ce{Fe^{2+}}\) would be ferrous ion and \(\ce{Fe^{3+}}\) would be the ferric ion.
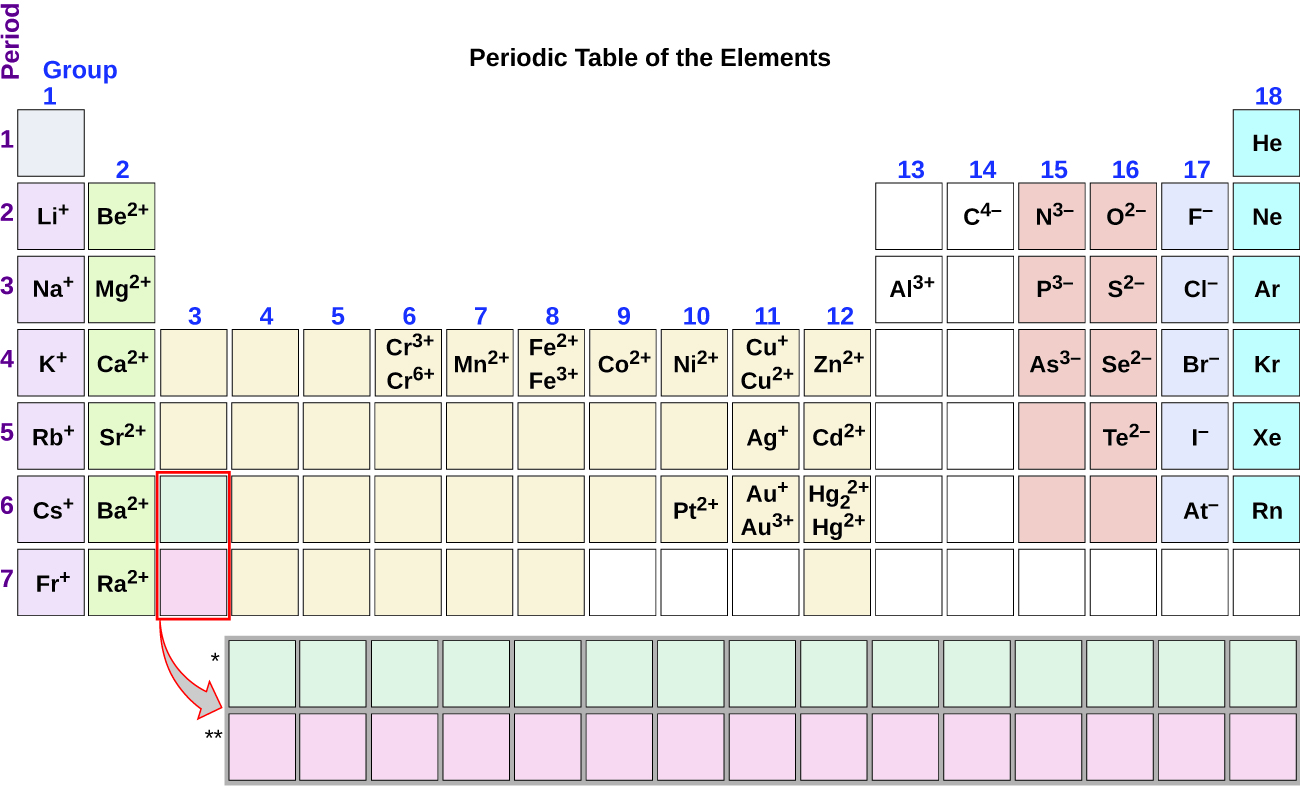
Common Charges of some monatomic ions.
Polyatomic Ions
When more than one type of atom is covalently bonded and has an overall charge, this is known as a polyatomic ion. When forming a stable crystal, these compounds have both covalent bonds (holding the polyatomic ion s together) and ionic bonds (holding the different ions together in a stable neutral lattice). Below is a list of polyatomic ions that you will be required to know.
Ionic Compound Formula and the Principle of Charge Neutrality:
The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that.
Trick:
Set # of Anions = Charge of Cation and
set # of Cations = Charge of Anion
Beware that this must be the lowest whole # ratio of cation to anion.
Strategy:
- Identify charge of each ion
- multiply charge of each ion by absolute value of the charge of the counter ion.
- Make sure resulting ratio is the lowest whole number ratio, if not, divide by common denominator so all values are integers.
What is the case when we write ionic formula that include polyatomic ions? The same rules above still apply. You must take note that parenthesis are needed around polyatomic ions if there is more than one polyatomic ion. Otherwise, there is no need for parenthesis. Watch this video for a tutorial.
Exercise \(\PageIndex{1}\)
Using the principle of Coulombs Law, would you expect \(KCl\) or \(\ce{Al2O3}\) to have a greater melting point?
- Answer
-
According to Coulombs Law, the greater the charge, the stronger the interaction between ions and the greater energy (temperature) needed to separate the ions.
Exercise \(\PageIndex{2}\)
Based on the position of the periodic table, predict the charge of the following elements if they are to produce ions. (do not look at periodic table above to come up with your answer)
- Sr
- Ga
- P
- Answer
-
A) \(\ce{Sr^{2+}}\). Sr is a group 2A main group metal. So, +2 charge.
B) \(\ce{Ga^{3+}}\). Ga is a group 3A main group metal. So, +3 charge.
C) \(\ce{P^{3-}}\). P is a group 5A main group nonmetal. So, 5-8 = -3 charge.
Exercise \(\PageIndex{3}\)
Name the following ions.
A) Al+3
B) Cu+2
C) N-3
D) NO3-
- Answer
-
A) monatomic cation with only one possible charge. Aluminum ion.
B) monatomic cation that can form more than one charge (usually transition metals) , so you need a Roman Numeral. Copper(II) ion.
C) monatomic anion. change the ending of nitrogen to the -ide ending. nitride ion.
D) polyatomic ion. nitrate ion. notice, the -ate and -ite ending is designated for polyatomic ions and the -ide ending is designated for monatomic ions.
Exercise \(\PageIndex{4}\)
Give the symbol and name for the ion with 34 protons and 36 electrons. Is it a cation or an anion?
- Answer
-
\(Se^{2−}\), the selenide ion. anion since there are more electrons than protons.
Exercise \(\PageIndex{5}\)
What is the formula for the ionic compound that forms when the calcium ion reacts with the oxide ion?
- Answer
-
\(CaO\). \(Ca^{2+}\) and \(O^{2-}\). You need one calcium ion for every one oxide ion for the compound to be charge neutral.
Exercise \(\PageIndex{6}\)
What is the formula for the ionic that forms when the lead(IV) ion reacts with the sulfate ion?
- Answer
-
Add \(\ce{Pb(SO4)2}\). \(\ce{Pb^{4+}}\) and \(\ce{SO4^{2-}}\). For every one Lead(IV) ion, you need two sulfate ions to be charge neutral. 1(+4) + 2(-2)=0. You may use the criss-cross rule, but you must make sure that you reduce to the simplest whole number ratio.