7.2: Electron Configurations of Atoms and Monatomic Ions
- Page ID
- 52824
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
In this section we shall assign electrons to orbitals and come up with some rules to determine the ground state electron configuration of atoms of the different elements. The ground state configuration is the lowest energy configuration, and the ground state electron configuration affects the behavior of atoms and results in the elemental structure of the periodic table. For example, all the alkali metals have "isoelectronic" valence shell electron configurations. That means their outer shells have similar numbers of electrons in similar orbitals, which for the alkali metals have a ns1 electron configuration, where n is the outer (valence) shell. Because of this they behave similarly, and for example, can easily lose the outer electron and form a cation of charge [+1].
It should be emphasized that the electron configurations we are describing are the ground state or lowest energy state configuration. In real systems atoms contain energy and electrons can exist in excited states, so you will have populations of atoms in different states, and these atoms will be perpetually changing states as they exchange energy with each other and the surrounding. At higher temperatures there will be more atoms in excited states. These atoms will relax back to the ground state, by either transferring thermal energy to other atoms or giving off a photon (radiative relaxation), and it was these radiative process that resulted in the atomic line spectra of the last Chapter.
Rule of Determining Electron Configurations
The following rules allow us to determine the ground state electron configuration, which is what we imply when we say "electron configuration". That is, unless otherwise stated, an electron configuration implicitly refers to the ground state. You first determine the number of electrons in the atom (or ion) and then assign them to orbitals according to the following principles or rules.
- Aufbau Principle
- Hunds Rule of Maximum Multiplicity
- Pauli Exlusion Priniciple
Aufbau Principle
Aufbau comes from the German "structure" or building up and Aufbauprinzip, is the "building up principle" where we assign electrons to the lowest energy orbitals first, and then successively add them to higher energy orbitals until we run out of electrons. This way the electron configuration is that of the lowest energy and so the Aufbau principle results in the ground state electron configuration. There are times when special attention needs to be given to the electron configurations and it may appear the Aufbau principle is not being obeyed, but it is. For example, it is for this reason that the 4s gets filled before the 3d, because as we noted in Figure 7.1.3 of section 7.1.2 the 4s orbital penetrates the 3d and although its average distance from the nucleus is greater than the 3d, when it's probability density is defined by the inner lobes of the wavefunction, it is closer to the nucleus and thus is of lower energy.
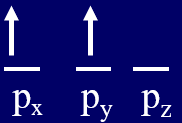
Hund's Rule
Hund's Rule of Maximum Multiplicity states that if you are filling degenerate orbitals that you put one electron in each before placing two electrons in one orbital, and they are assigned parallel spin (left configuration of Figure \(\PageIndex{1}\)). There are two factors going on here. First, electron-electron repulsion will cause the orbitals to be as far apart as possible, and so the configuration on the right part of Figure is the highest energy because the electrons are in the same orbital. The image on the left is lower in energy than the middle one due to magnetic interactions (you can visualize the arrow to point in the direction the magnetic force would interact). In the middle image the two magnetic fields are in opposite directions and so are working against each other, making that a higher energy state. In fact, if you place two identical magnets on top of each other (right most image) they will cancel each other out. The image on the left is the lowest energy because the are both far apart and the magnetic fields of the intrinsic spin of the electron align, and so that is the ground state. The other two states are allowed, and are called excited states because they are at a higher energy level than the ground state.
 |
 |
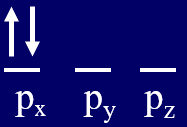 |
Figure \(\PageIndex{1}\): Three possible spin alignments for placing two electrons in the three degenerate p orbitals. The configuration on the left is the lowest energy state, the two on the right are allowed states, but are "excited states."
Hunds Rule is called Hunds Rule of Maximum Multiplicity because the spectral lines on the left configuration of Figure \(\PageIndex{1}\) will split into three lines in the presence of an external magnetic field. This is because for an ensemble of atoms some will have their magnetic moments perpendicular to an external magnetic field (no effect), some aligned with it, and others aligned against it, and Hunds rule states that the electron configuration that has maximizes the number of lines is the lowest energy state. So spin states of electrons can be seen through their interactions with magnetic fields and this shows in their spectra.
Note, there is a fourth possible configuration, which is with both electrons in the same orbital with the same spin, and that is not allowed because of the Pauli Exclusion principle.
Pauli Exclusion Principle
The Pauli Exclusion Principle states that no two electrons can have the same set of 4 quantum numbers, and thus if two electrons are in the same electronic they must have opposite spin quantum numbers
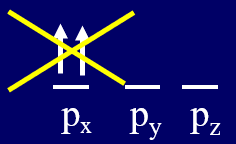
Figure \(\PageIndex{2}\): A forbidden electron configuration due to the Pauli Exclusion Principle
Note, the three configurations of Figure \(\PageIndex{1}\) are allowed, with the left-most one correlating with the ground state, while the configuration on \(\PageIndex{2}\) is forbidden and not allowed. So Hund's rule tells you which of these allowed states is lowest in energy, while the Pauli Exclusion principle defines configurations that can not exist, and excludes them as options.
Electron Configurations
Starting with the first row we get the following electron configurations for hydrogen and helium, noting how the coupled electrons in helium are spin paired (in opposite directions) according to the Pauli Exclusion principle
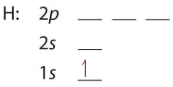 |
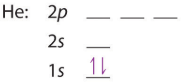 |
| H: 1s1 | H: 1s2 |
For the second period we start by filling the 2s orbitals, now the first shell is a core shell and the second is the valence, and we often use the Nobel gas to indicate the core electron configuration (which is that of Helium in this case). This second convention is often not used in the second period, but is used extensively when the number of core electrons becomes excessive.
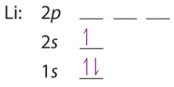 |
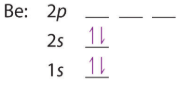 |
| Li: 1s22s1 or [He]2s1 | Be: 1s22s2 or [He]2s2 |
After filling the 2s the 2p are filled, in accordance with the Aufbau principle and Hund's rule.
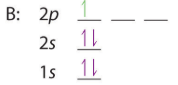 |
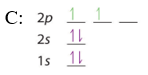 |
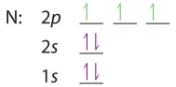 |
| B: 1s22s22p1 or [He]2s22p1 | C: 1s22s22p2 or [He]2s22p2 | N: 1s22s22p3 or [He]2s22p3 |
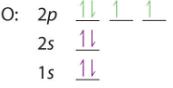 |
 |
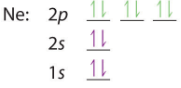 |
| O: 1s22s22p4 or [He]2s22p4 | B: 1s22s22p5 or [He]2s22p5 | B: 1s22s22p6 or [He]2s22p6 |
We note above that in accordance with Hund's rule each of the degenerate p orbitals were given 1 electron until they were all filled, and we started pairing up electrons with oxygen. The next element would be sodium, the alkali metal of the third period. With an atomic number of 11 sodium has 10 electrons and at this point the value of using the shorthand notation where a Nobel gas represents the core electrons is of obvious values. That is, you could write Na: 1s22s22p63s1 or [Ne]3s1 noting that [Ne]=1s22s22p6.
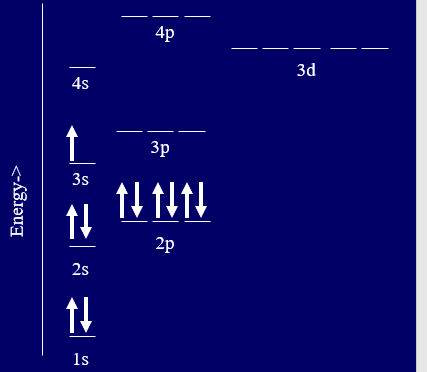
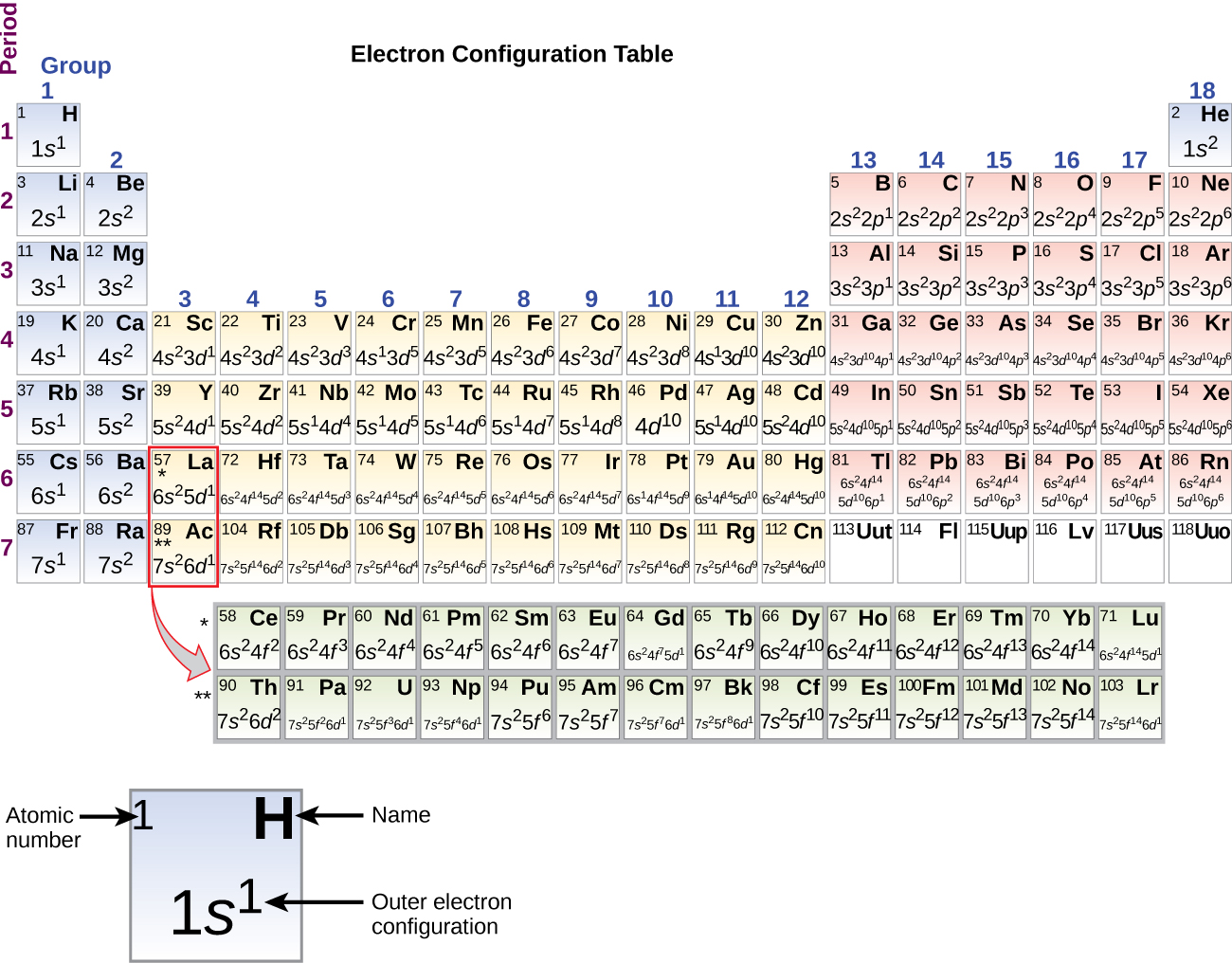
Lets look at the electron configurations of the representative elements (s and p block)
From table \(\PageIndex{1}\) [ ] indicates core electrons (not noble gas configuration) and it is clear that elements of a family have similar electron configurations for the outer shell, just a different principle quantum number.
Special Cases
Some of the transition elements have electron configurations that are not what you would expect based on the material presented in section 7.1.
Chromium: \[Cr: \;[Ar]4s^13d^5\]
This may seen strange, but if you look at the spin pairing energy all 6 spins are aligned, while if you just walked through the pattern, you would come up with \(4s^23d^4\) and what is going on is that the gap between the 4s and 3d is so small that the spin pairing energy is greater. Also, this configuration is entirely symmetric which also influences its stability (there are no empty orbitals and they all have the one electron). The same logic goes for Molybdenium
Molybdenium: \[Mo: \;[Kr]5s^14d^5 \]
Copper and Silver also show these kind of anomalies
Copper: \[Cu: \; [Ar]4s^13d^{10}\]
Silver: \[Ag: \; [Kr]5s^14d^{10}\]
Here there is extra stability being gained by having a completely filled subshell.
Note
UALR students are expected to know the electron configurations for chromium, molybdenium, copper and silver, and give a rough explanation of why the are not as would have been predicted if we just blindly followed the rules outline above.
Palladium has a filled 4d and empty 5s while Nickel has the configuration you would expect
Palladium: \[Pd: \; [Kr]4d^{10}\]
Nickel: \[Ni: \; [Ar]4s^23d^{8}\]
Niobium is also not as would be predicted, and once again this is dealing with the fact that the 5s and 4d orbits are real close in energy, and the gap is of the order of the spin pairing energy.Niobium: \[Nb: \; [Kr]5s^14d^4\]
Exercise \(\PageIndex{1}\)
Write the ground state electron configuration of yttrium in both the long format and the short nobel gas format.
- Answer
-
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1
[Kr]5s2 4d1
Exercise \(\PageIndex{2}\)
Write the electron configurations of:
- Argon
- Krypton
- Radon
- Silver
- Answer a
-
[Ne] 3s2 3p6
- Answer b
-
[Ar] 4s2 3d10 4p6
- Answer c
-
[Xe] 6s2 4f14 5d10 6p6
- Answer d
-
[Kr] 5s1 4d10
Exercise \(\PageIndex{3}\)
How many unpaired electrons are in:
- Vanadium
- Chromium
- Iron
- Copper
- Answer a
-
Three in the 3d orbitals ([Ar] 4s2 3d3)
- Answer b
-
six, 5 in the 3d and one in the 4s ([Ar] 4s1 3d5)
- Answer c
-
four, in the 3d ([Ar] 4s2 3d6)
- Answer d
-
one in the 4s ([Ar] 4s1 3d10)
Electron Configurations of Ions
Anions
Electron configuration of anions is pretty straight forward. You simply have additional electrons to the neutral atom, and add them the same way you would for an atom. Note, although monatomic anions are isoelectronic to a nobel gas, (chloride has the same electron configuration as Argon), you write down the electrons of its valence shell. So chloride's configuration is Cl-: [Ne]3s23p6 and not [Ar].
Cations
For cations you need to be careful and the electron comes from the highest principle quantum shell. So calcium and Titanium(II) have the same number of electrons, but they have different electron configurations (they are not isoelectronic).
Calcium (20 electrons): \[Ca: \; [Ar]4s^2 \]
Titanium(II) (20 electrons): \[Ti^{+2}: \; [Ar]3d^2\]
The reasoning can be seen by the radial wavefunction density plot (Figure \(\PageIndex{5}\)).

Figure \(\PageIndex{5}\): You note the 4s orbital has density that is both closer and farther from the nuclues than the 3d. For this reason the 4d gets filled before the 3d when building up an electron configuration along the lines of the aufbau principle. But when you remove an electron to form a cation, the 42 is also farther away from the nuclues, and so is easier to remove. This is a consquence that an electronic orbital is described by a wavefunction. (image cc 4.0 from chemistry stack exchange uploaded by orthocresol)
Exercise \(\PageIndex{4}\)
Using noble gas shorthand notation, write the electron configurations of:
- chloride
- potassium cation
- iron (II) and iron (III) (ferrous and ferric)
- lead(II) and lead (IV) (plumbous and plumbic)
- Answer a
-
[Ne] 3s2 3p6
- Answer b
-
[Ar]
- Answer c
-
[Ar] 3d6 and [Ar] 3d55
- Answer d
-
[Xe] 6s2 4f14 5d10 and [Xe] 4f14 5d10
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
Some material adopted, modified or adapted from
- anonymous contributors to LibreText
- Ronia Kattoum and November Palmer


